- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Effect of H. pylori Infection on Hematological Parameter among Patient with H. pylori Infection at Atbara City 2017
Mohammed Hashim Fadellala1, Abuzar Elnager1, Maha Khalifa Elkhair2 and Musab Nouraldein Mohammed3*
1 Department of Hematology and Blood Transfusion, Faculty of Medical Laboratory Sciences, Sudan
2 Atbara Teaching Hospital, Sudan
3 Department of Medical Laboratory Sciences, Sudan
*Corresponding author: Musab Nouraldein Mohammed, Department of Medical Laboratory Sciences, Sudan
Submission: September 27, 2018;Published: December 14, 2018

Volume2 Issue2 December 2018
Abstract
H. pylori is one of common infection of human. It is associated with chronic gastritis, peptic ulcer, mucosa related tissue lymphoma and gastric cancer. The platelets indices (MPV, PDW and PLCR) used as indicator of systemic inflammation. This is cross sectional study conducted in Atbara city from patient with H. pylori and compared with normal control groups according to selection Criteria. Venous blood (2.5ml) were collected in EDTA containers from (100) patient with H. pylori and (100) subject as normal control groups the platelets indices, neutrophils and lymphocytes were measured using Sysmex KX-21N analyzer. The data was analyzed by SPSS software using Independent test. The result of this study showed that mean SD of H. pylori patient neuterophils, lymphocytes, MPV, PDW and PLCR 55.9, 31.46, 8.9, 10.03 and 17.47 respectively. There was a significant mean difference between MPV, PDW and PLCR (P< 0.05). However, there was insignificant MD between H. pylori patient and normal control groups for neutrophil and lymphocyte count (p< 0.05). there was association between MPV, PDW and age among H. pylori patients r2=0.005 however there was negative association between PLCR and age among H. pylori patients r2=0.008. The study concluded that platelets indices in Atbara city from patient with H. pylori and normal control groups There was insignificant neutrophil and lymphocyte count but there as correlation to age in MPV, PDW but not PLCR. .
Keywords: H. pylori infection; Hematological parameters
Abbreviations: TNF: Tumor Necrosis Factor; MPV: Mean Platelet Volume; PDW: Platelet Distribution Width; P-LCR: Platelet Large Cell Ratio; PCT: Platelet-Crit
Introduction
Literature review
Helicobacter pylori infection is the most common bacterial infection Worldwide, with almost half of people in developed countries and three-quarters of people in developing countries infected. Although many infected individuals are asymptomatic, H. pylori is an important health problem [1]. Prevalence of H. pylori depends on geographical location and socioeconomical status but approximately 50% of the world adult population is infected by H. pylori [1].
Gastric mucosa it generally does not invade the gastroduodenal tissue but triggers a host immune response which leads to tissue injury. Although it is a noninvasive organism itself, the antigenic substances it produces such as heat shock protein, urease and lipopolysaccharide activate T cells. With an enhanced antigen presentation, inflammatory cytokines such as IL-1, IL-6, IL-8, cytokines such as IL-1, IL-6, IL-8, tumor necrosis factor-alpha (TNFalpha) are released. Besides, both local and systemic B cell response also occurs. Through these local and systemic immune responses, H. pylori is known as a causative agent for gastritis, peptic ulcers and probably by a chronic infection and immune stimulation, gastric cancer and mucosa-associated lymphoid tissue lymphoma. It has also been associated with diseases outside the gastrointestinal tract such as coronary heart disease, acne rosacea, chronic idiopathic urticaria, iron deficiency anemia and systemic autoimmune diseases [2]. Existence of H. pylori can be determined by noninvasive tests such as anti H. pylori antibodies in the blood or urea breath test in patients with symptoms of dyspepsia [3].
H. pylori is linked to ITP with an unclear mechanism. One probable explanation is molecular mimicry; an antibody induced by H. pylori may cross react with platelet glycoprotein antigens. Cag A positive strains are also suggested as pathogen- (i.e. causatives for ITP). One other probability is the enhancing effect of H. pylori on already present antiplatelet antibodies and platelet phagocytosis. Genetic factors of the host are also reported to provide a susceptibility for H. pylori related platelet destruction [4]. Eradication of H. pylori has been associated with a platelet response, to a degree and in certain group of patients. Though still bearing controversy, this treatment is mentioned in ITP management guidelines [5].
Colonization with H. pylori is not a disease in and of itself, but a condition associated with several disorders of the upper gastrointestinal tract [6]. Testing for H. pylori is recommended if peptic ulcer disease or low-grade gastric MALT lymphoma is present, after endoscopic resection of early gastric cancer, first-degree relatives with gastric cancer, and in certain cases of dyspepsia [7], not routinely [8]. Several ways of testing exist. One can test noninvasively for H. pylori infection with a blood antibody test, stool antigen test, or with the carbon urea breath test (in which the patient drinks 13C-or 14C-labelled urea, which the bacterium metabolizes, producing labeled carbon dioxide that can be detected in the breath) [9]. Also, a urine ELISA test with a 96% sensitivity and 79% specificity is available. None of the test methods is completely failsafe. Even biopsy is dependent on the location of the biopsy. Blood antibody tests, for example, range from 76% to 84% sensitivity. Some drugs can affect H. pylori urease activity and give false negatives with the urea-based tests. The most accurate method for detecting H. pylori infection is with a histological examination from two sites after endoscopic biopsy, combined with either a rapid urease test or microbial culture [10].
Platelets are cytoplasmatic fragments of bone marrow megakaryocytes, with a diameter of 3-5μm and a volume of 4.5- 11fL [11]. Single megakaryocytes release 1500-2000 of them to the blood stream, where they circulate for 7-10 days. Inactivated platelets in the blood are discoid shaped and do not contain a nucleus. Their cytoplasm contains three different types of granules (i.e. alpha granules, dense granules, and lysosomal granules), secretory vesicles that contain pre-formed molecules, and a complex membranous system.
Platelets have essential roles in thrombosis and hemostasis. The normal platelet count ranges hemostasis. The normal platelet count ranges from 100×109/L to 450×109/L. Circulating lifespan of platelets is 10 days and almost one third of platelets are sequestered in the spleen. Approximately 100×109 of platelets are released from megakaryocytes every day to maintain the adequate platelet count. Therefore, a regular and continuous balance between production and consumption is required. Generally defined as platelet count less than 150×109/L, thrombocytopenia is caused by increased destruction or consumption, splenomegaly, and decreased production due to bone marrow suppression or failure. Besides the contribution of genetic factors, age, gender and seasonal variations are known to affect the platelet count [12]. Existence of H. pylori can be determined by noninvasive tests such as anti-H pylori antibodies in the blood or urea breath test in patients with symptoms of dyspepsia. But these tests do not give us any information about intensity of H pylori in gastric mucosa and severity of acute and chronic inflammation. We aimed specially to investigate the usability of MPV as a simple indicator that may reflect severity of inflammation in gastric mucosa [13].
Platelet size is a useful indicator to differentiate between hypo proliferative & hyper destructive thrombocytopenia. The combined interpretation of platelet (PLT) count, platelet-Crit (PCT), Mean Platelet Volume (MPV), Platelet Distribution Width (PDW) & Platelet Large Cell Ratio (P-LCR) by automated cell counters can be very useful parameters in differentiating thrombocytopenia due to various etiologies. Platelet count in the blood can be rapidly measured using an automated hematological analyzer. Platelet indices are biomarkers of platelet activation. They allow extensive clinical investigations focusing on the diagnostic and prognostic values in a variety of settings without bringing extra costs. Among these platelet indices, platelet-crit (PCT), mean platelet volume (MPV), and platelet distribution width (PDW) are a group of platelet parameters determined together in automatic CBC profiles; they are related to platelets morphology and proliferation kinetics.
An increase in both mean platelet volume (MPV) and platelet distribution width (PDW) due to platelet activation was suggested due to:
a. Platelet swelling.
b. Pseudopodia formation.
c. MPV and PDW are simple platelet indices, which increase during platelet activation
Mean platelet volume (MPV)
In MPV, the analyzer-calculated measure of thrombocyte volume is determined directly by analyzing the platelet distribution curve, which is calculated from a log transformation of the platelet volume distribution curve, to yield a geometric mean for this parameter in impedance technology systems. In some optical systems, MPV is the mode of the measured platelet volume [14]. The most commonly available derived parameter is the mean platelet volume (MPV), calculated by dividing the platelet-crit (PCT) (Platelet-crit is a measure of total platelet mass. Values vary depending on mean platelet volume resulting in overlap between normal platelets, thrombocytopenia and thrombocytosis). By the total number of platelets. This is analogous to the calculation for the mean red cell volume (MCV), in which the hematocrit is divided by the total red cell count.
Is average size of the platelets in Blood Normal range of MPV=(7.5-11.5fl). Usually MPV>13 occurs in hyper- destruction & MPV< 8 in hypo-production of platelet. When platelet production is decreased, young platelets become bigger and more active, and MPV levels increase. Increased MPV indicates increased platelet diameter, which can be used as a marker of production rate and platelet activation. During activation, platelets’ shapes change from biconcave discs to spherical, and a pronounced pseudopodia formation occurs that leads to MPV increase during platelet activation. Widely used marker of platelet function, mean platelet volume (MPV), is affected from the production step through activation and finally the production step through activation and finally sequestration. In patients with thrombocytopenia, platelet volume analysis [14].
May be practical in differential diagnosis. Platelet size does not depend on its age. It is rather determined as they are produced from the megakaryocytes as pre-platelets. Like their size and shape, their cytoplasmic characteristics are determined by the megakaryocytes from which they arise. Large and big platelets are more active and tend to aggregate. This type of platelets contains denser granules, secretes more serotonin and thromboglobulin and produces more thromboxane A2 compared to small platelets [15].
Recently, mean platelet volume (MPV) has been started to be used as a simple inflammatory indicator in some diseases. Normal MPV values are 7.2 to 10.1fL. It has been observed in some studies that MPV level increased in myocardial infarction and cerebrovascular diseases [16]. Mean platelet volume (MPV) has been started to be used as a simple inflammatory indicator in some diseases. High MPV values indicate larger and more active platelets which contribute to the thrombotic events. Some studies yielded higher MPV values for diseases assumed to have low grade inflammation such as diabetes mellitus and coronary artery disease. It has been observed in some studies that MPV level increased in myocardial infarction and cerebrovascular diseases. However, it has been reported that MPV level has decreased in active rheumatic diseases such as rheumatoid arthritis and any losing spondylitis [17]. Similarly, in another study, reduced platelet volume in patients with ulcerative colitis was determined [18].
Platelet distribution width (PDW)
Is an indication of variation in platelet size which can be a sign of active platelet release the PDW median and was 13.3%, with a reference range of 10.0%-17.9% for the 5th-95th percentiles, with a confidence interval of 95. The PDW is a reasonably good tool to distinguish essential thrombocythemia (PDW increased) from reactive thrombocytosis (PDW normal) [19]. PDW is a distribution curve of platelets measured at the level of 20% relative height in a platelet-size distribution curve, with a total curve height of 100. PDW directly measures variability in platelet size, changes with platelet activation, and reflects the heterogeneity in platelet morphology indication of variation in platelet size which can be a sign of active platelet release the PDW median [20].
Platelet larger cell ratio (P-LCR)
Is an indicator of circulating larger platelets (>12fL), which is presented as percentage? The normal percentage range is males 12.7-36.1%, females 10.9-38.8%. It has also been used to monitor platelet activity [21].
Complete blood count (CBC)
A complete blood count (CBC) is a blood test used to evaluate the overall health and diagnose a wide range of disorders, including anemia, infection, leukemia etc. Sysmex Automated Hematology Analyzer XT-2000i advanced instrument was used for CBC and platelet indices. The specific parameters included in the study were neutrophils, lymphocytes, MPV, PDW, P-LCR, and PLT-crit [22].
General studies has been done regarding the effect of H. pylori on hematological parameters among patient A study done by Lewis SM, -2006 Mean platelet volume: a useful marker of inflammatory bowel disease activity reported that no significant witch agreement study [23]. Another study was associated of severity of H.P infection with peripheral neutrophil/lymphocyte ratio and MPV concluded that moderate increase intensity of H.P does not lead to significant change in MPV [24].
A study in Turkey, concluded that H. pylori infection and normal platelet counts, it may be speculated that an ongoing and compensated platelet destruction-production may be responsible for the increase in MPV [25]. Other study [26] performed in MPV only, which reported there no relationship to the severity of the H.pylori according to the lymphocyte and neutrophil [26]. Other Turkey study [2010] concluded there were no correlation between MPV and HP intensity, acute and chronic inflammation [27].
Rationale
H. pylori is an important health problem. The prevalence of H. pylori depends on geographical location and socioeconomical status but approximately 50% of the world adult population is infected by H. pylori. Platelet indices as inflammatory indictor which help for diagnosis of many disease such as rheumatoid arthritis, myocardial infarction and bowel disease. There is limited, or none publish data regarding the Platelet indices and it is relation with H. pylori. Therefore, this study was conducted to determine the relationship between Platelet indices and H. pylori infection.
Objectives
General objective
To determine the Effect of H. pylori infections on platelet indices among patients with H. pylori infections in Atbara city 2017.
Specific objectives
A. To estimate the platelet indices (MPV, PRW and P-LCR) among the patient with H. pylori infection and normal control in Atbara city using SYSMEX KX21N.
B. To compare the result of platelet indices between the H. pylori patients and normal control group.
C. To know the effect of age and gender on and platelets indices H. pylori infected people.
D. To count lymphocytes and neutrophils in H. pylori patients and normal control.
E. To compare of lymphocyte and neutrophils of H. pylori patients to the normal control.
F. To know the effect of H. pylori infection on lymphocytes and neutrophils.
Materials and Methods
Study design?
Cross sectional study.
Study area
Atbara city.
Study duration
From August-October2017.
Study population
H. pylori positive patient and healthy control group.
Ethical consideration
The individuals included in the study were notified well about the objective and the need of this study and they accept to give blood samples before the start of the collection process.
Sample size formula
Sample size was 100 sample H.P patient and 100 sample healthy control group
Selection criteria
Inclusion criteria:
1. Live in Atbara locality.
2. Positive H. pylori for case and negative H. pylori for control group.
3. From both gender and any age.
Exclusion criteria: For control group diabetes mellitus, coronary artery disease, renal failure, liver failure, cardiac failure, inflammatory disease such as rheumatoid arthritis, systemic lupus erythematosus and malignancies.
Data collection: The primary data was collected by a standard questionnaire and the secondary data was analyzed.
Blood sample collection
First, the patient identity has been investigated for ICT for H. pylori and found that it corresponds to the details on request form. Recommended that skin must be cleaned by 70% alcohol or by 0.5% chlorhexidine and allowed to dry before being punctured .Blood best withdrawn from antecubital vein or other visible vein in the forearm by either an evacuated tube or a syringe. The tourniquet has been applied just above the venipuncture site and released as soon as the blood has been flown into syringe or evacuated tube. If a syringe was used for blood collection the piston of syringe should be withdrawn slowly, Anticoagulated specimen was mixed by harvesting the containers several times. After the blood was obtained and released the tourniquet, the needle was removed and then pressed applying pressure on the swab, Then the punctured site was covered with a small adhesive dressing.
Test procedure
The MPV is calculated by the following formula: MPV (fl)= ((platelet (%) / Platelet count (x109/l)) [22]. PCT is the ratio of the platelet volume in relation to blood volume. PDW is the width of the size distribution curve in femtoliter fl). An increase in both mean platelet volume (MPV) and platelet distribution width (PDW) due to platelet activation, and platelets destruction. The P-LCRindicator of circulating larger platelets (>12 fL), which is presented as percentage.
6.10. Data analysis: Data was analyzed by SPSS variation 22 using an Independent t test and simple linear regreen.
Results
A total of (200) subject was enrolled in this study (100) subjects positive H. pylori and (100) normal control groups, the patients and normal control groups were selected according to inclusion criteria in Atbara city. The mean age was 38 years old Table (3.1) shows the comparison of mean (SD), mean difference, lower and upper 95% confidence interval of neutrophils, lymphocytes count, platelets indices (MPV, PDW and PLCR) between H. pylori patient and normal control groups. There was a significant mean difference between MPV, PDW and PLCR (P< 0.05). However, there was insignificant MD between H pylori patient and normal control groups for neutrophils and lymphocytes count (p< 0.05). These results showed reflect the effect noise on the above hematological parameters among subjects exposed to noise Figure 1-3 and Table1.
Table 1:Comparison of mean difference of hematological parameters between H. pylori patients and normal control n=50.
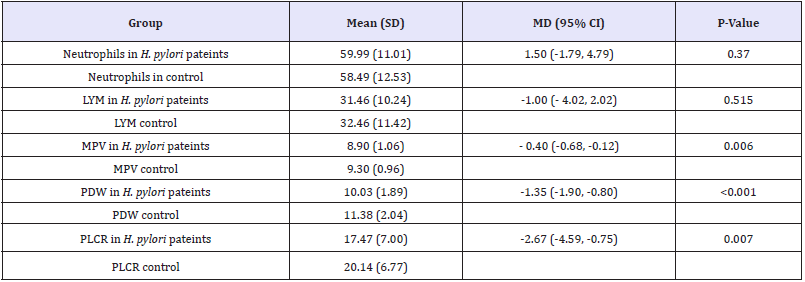
Independent t. test was applied. P-value as 0.05 is significant.
Figure 1:shows positive association between MPV and age among H. pylori patients r2=0.005. It shows the relationship between MPV level and age among H. pylori patients in Atbara city (n=40)
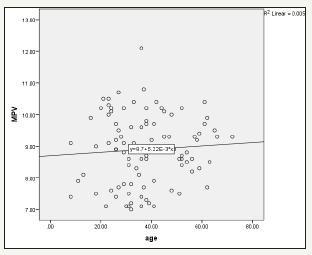
Figure 2:shows positive association between PDW and age among H. pylori patients r2=0.001. It shows the relationship between PDW level and age among H. pylori patients in Atbara city (n=40)
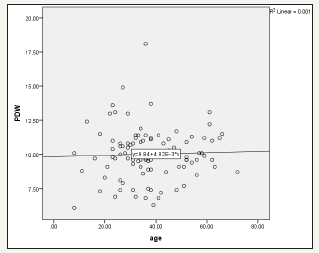
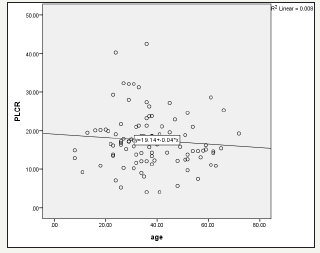
Figure 3:The relationship between PLCR level and age among H. pylori patients. It shows negative association between PLCR and age among H. pylori patients r2=0.008
Discussion
Helicobacter pylori infection is the most common bacterial infection Worldwide, with almost half of people in developed countries and three-quarters of people in developing countries infected. Although many infected individuals are asymptomatic, although the relation of H. pylori and ITP is thoroughly investigated, MPV has been the subject of only 5 small studies. These studies focused on the practicability of MPV in the prediction of H. pylori infection intensity and observed no correlation. However, they have not referred to the effect of H. pylori infection on platelet counts or MPV.
A study done by Lewis SM, -2006 Mean platelet volume: a useful marker of inflammatory bowel disease activity reported that no significant between MPV and inflammatory bowel disease which agree with our study [24]. Another study was associated of severity of H.P infection with peripheral neutrophil/lymphocyte ratio and MPV concluded that moderate increase intensity of H.P does not lead to significant change in MPV which agreement with our study [25].
A study in Turkey, concluded that H. pylori infection and normal platelets count, it may be speculated that an ongoing and compensated platelet destruction-production may be responsible for the increase in MPV [26]. Other study in Turkey1996 performed in MPV only, which reported there no relationship to the severity of the H. pylori according to the lymphocyte and neutrophil and this result was agreed to this study. [27]. Other Turkey study (2010) concluded there was no correlation between MPV and HP intensity, acute and chronic inflammation which agreement to this study.
The report of this study showed that measure (SD) of Neutrophils 59.99 lymphocyte 31.46, MPV 8.9, PRW 10.0, P-LCR17.47, respectively. There is insignificant variant for Neutrophil and lymphocyte for the H. pylori infection in this study. The platelets indices which reflect the effect on H. pylori. There are no significant differences among the genders with the platelet indices.
Conclusion
This study concluded that H. pylori had little effect on platelets indices and there was no effect to of H. pylori on lymphocytes and neutrophils.
Recommendation
We recommend that other studies with large sample size should be done to exclude or confirm the effect of H. pylori on platelets indices and lymphocytes and neutrophils.
Acknowledgment
Firstly, my great thanks to Allah for guiding me to the straightway in my life. Many thanks and appreciations are extended to my teachers for their support. To Dr. Abuzar Elnager for his valuable advice and endless efforts to make this work came into reality.
References
- Cave DR (1996) Transmission and epidemiology of Helicobacter pylori. Am J Med 100.
- Pounder RE, Ng D (1995) The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther.
- Traci L Testerman, James Morris (2014) Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol 20(36): 12781-12808.
- Stasi R1, Provan D (2008) Helicobacter pylori and chronic ITP. Hematology Am Soc Hematol Educ Program pp: 206-211.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L et at. (2011) Crowther MA. The American Society of Hematology 2011 evidencebased practice guideline for immune thrombocytopenia. Blood 117(16): 4190-4207
- Kusters JG, van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori Infection. Clin Microbiol Rev 19(3): 449-490.
- Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347(15): 1175-1186.
- Senaran H, Ileri M, Altinbas A, Kosar A, Yetkin E, et al. (2001) Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol 24: 405-408.
- Bussel J, Cines D (2005) Immune thrombocytopenic purpura, neonatal alloimmune thrombocytopenia. In: Ronald Hoffman, Edward J (Eds.), Hematology: Basic Principles and Practices. (4th edn), Churchill Livingstone, London, UK, pp.1321-1313.
- Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR (1982) Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol 50: 509- 519.
- Bath PM1, Butterworth RJ (1996) Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 7: 157-161.
- Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, houston 1994. Am J Surg Pathol 20: 1161-1181.
- Bath PM, Butterworth RJ (1996) Platelet size: Measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 7: 157-161
- Endler G, Klimesch A, Sunder-Plassmann H, Schillinger M, Exner M, et al. (2002) Mean platelet volumeis an independent risk factor for myocardial infarction but not for Coronaryartery disease. Br J Haematol 117: 399-404.
- Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, et al. (2001) Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol 96(3): 776-781.
- Dastjerdi MS, Emami T, Najafian A, Amini M (2006) Mean platelet volume measurement EDTA or citrate? Hematology 11(5): 317-319.
- Stenström B, Mendis A, Marshall B (2008) Helicobacter pylori-The latest in diagnosis and treatment. Aust Fam Physician 37(8): 608-612.
- Logan RP, Walker MM (2001) Epidemiology and diagnosis of Helicobacter pylori infection. BMJ 323: 920-922.
- Sachdev R, Tiwari AK, Goel S, Raina V, Sethi M, et al. (2014) Establishing biological reference intervals for novel platelet parameters (immature platelet fraction, high immature platelet fraction, platelet distribution width, platelet large cell ratio, platelet-X, plateletcrit, and platelet distribution width) and their correlations among each other. Indian J Pathol Microbiol 57: 231-235.
- Hong H, Xiao W, Maitta RW (2014) Steady increment of immature platelet fraction is suppressed by irradiation in single-donor platelet components during storage. PLoS One 9(1).
- Bath PM, Butterworth RJ (1996) Platelet size: Measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 7(2): 157-161.
- TibebeAdinew (2015) Performance Evaluation of Sysmex KX-21 and Cell-Dyn 1800 Hematology Analyzers at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia.
- Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, et al. (2008) Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine 75: 291-294.
- Lewis SM, Bain BJ, Bates I (2001) Dacie and Lewis Practical Haematology. In: Kapsoritakis AN, Koukourakis MI, Sfiridaki A. (10th edn), Mean platelet volume: a useful marker of inflammatory bowel disease activity. Churchill Livingstone 2006, Philadelphia, Am J Gastroenterol 96: 776- 781.
- Kaito K, Otsubu H, Usui N, Yoshida M, Tanno J, et at. (2005) Platelet size deviation with, platelet large cell ratio, and mean platelets volume have enough sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br J Haematol 128: 698-702.
- Järemo P, Sandberg-Gertzen H (1996) Platelet density and size in inflammatory bowel disease. Thromb Haemost 75(4): 560-561.
- Topal F, Karaman K, Akbulut S, Dincer N, Dolek Y, et at. (2010) The relationship between mean platelet volume levels and the inflammation in Helicobacter pylori gastritis. J Natl Med Assoc 102(8): 726-730.
© 2018 Musab Nouraldein Mohammed. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






