- Submissions

Full Text
Advances in Complementary & Alternative medicine
Effect of Suo Quan Wan on Bladder Function and Cx43 Expression in Guinea Pigs with Bladder Outlet Obstruction
Weiwen Jiang1, Huanling Lai1,2, Bo Tan3, Yifei Xu1, Pinglong Fan1, Dawei Lian1, Lijun Fu1, Wenkang Ren1, Qinghe Wu1, Ping Huang1* and Hongying Cao1*
1School of Chinese Materia Medical, China
2State Key Laboratory of Quality Research in Chinese Medicine, China
3School of Fundamental Medical Science, China
*Corresponding author: Hongying Cao, School of Chinese Materia Medica, China Ping Huang, School of Chinese Materia Medica, China
Submission: January 09, 2019;Published: January 22, 2019

ISSN: 2637-7802 Volume3 Issue4
Abstract
Context: Suo Quan Wan (SQW) is an effective traditional Chinese prescription for treating lower urinary tract symptoms and exhibits modulatory effects on TRPV1 and P2X3 expression and adenosine triphosphate (ATP) release in accordance with the recovery of bladder function in overactive bladder (OAB) rat. This interaction between cells of the bladder is relayed to gap junction channels.
Objective: This experiment further investigates the mechanism of SQW in modulating gap junction channels by Cx43.
Materials and Methods: This study was carried out using a guinea pig OAB model created by bladder outlet obstruction (BOO) surgery. The model was divided into the model group and SQW-treated group with the sham operation group as control. In vivo study with voided stain on paper test was performed to evaluate the micturition behavior of guinea pigs, and urodynamic assessment was conducted to measure the differences in bladder functions of each group. The bladder weight to body weight ratio was also analyzed. Then, real-time polymerase chain reaction and Western blot were performed to quantify TRPV1 expression in the bladder. An In vitro organ bath was used to study bladder distension response to various compounds.
Results: The guinea pigs received BOO surgery after 3 weeks and exhibited significantly increased micturition times, bladder pressure (BP) and capacity, and postvoiding residual. Meanwhile, voiding efficiency (VE) was decreased. In vitro study showed increased force response to α, β-me-ATP, KCl, and carbachol and high expression level of Cx43 on the bladder. The bladder weight to body weight ratio was also increased. SQW treatment can improve bladder function by modulating BP and increasing VE to reduce postvoiding residual, which decreases micturition times. Cx43 expression was decreased. Force response to α, β-me ATP, KCl, and carbachol was decreased.
Conclusion: SQW can modulate Cx43 expression in accordance with the recovery of bladder function.
Keywords: Suo Quan Wan (SQW); Intracellular communication; Connexin 43; Overactive bladder; Outlet obstruction
Introduction
Urgency, with or without incontinence, usually with increased daytime frequency and nocturia and absence of infection, is the characteristic symptom of overactive bladder (OAB) in the clinical setting, as defined by the Standardization Sub-committee of the International Continence Society [1]. OAB is characterized by complicated symptoms and increasing morbidity. However, its mechanism has not yet been completely revealed. For normal bladder function, the intracellular communication between the smooth muscle cells is necessary for tissue coordination, especially the detrusor muscle [2]. Gap junction proteins are essential for the direct exchange of small molecules including adenosine triphosphate (ATP) and cyclic adenosine monophosphate between adjacent cells. Altered intracellular communication is found to be involved in OAB pathophysiology. In this aspect, connexin proteins, such as Cx26, Cx40, Cx43, and Cx45, are suggested to be part of the coordinated mechanism of detrusor cells and have been identified in stable bladders of humans and other animals [3-5]. The altered connexin-43 expression level in the bladder is considered to be the underlining mechanism of OAB conditions caused by various pathologies. High expression levels of Cx43 are related to overactive neurogenic detrusor [6], whereas decreased levels of Cx43 are related to an underactive bladder [7].
As natural products are effective and have low toxicity, they have been studied and used worldwide as potential agents for treating various diseases. For example, Suo Quan Wan (SQW), a common traditional Chinese medicine, comprising Alpinia oxyphylla Miq, Dioscorea rhizome Thunb., and Aconiti tuber, has been used to treat various urinary system diseases in China for a long time. Our previous study proved that SQW has certain therapeutic properties that nourish the kidney and reduce urination by increasing the expression levels of AQP2, AVPR-V2 [8], and CYP11B2 and also serum levels of Cort and ALD in kidney deficiency polyuria rats [9]. Moreover, our previous study with OAB model rats showed that SQW treatment can significantly reverse the increasing bladder pressure (BP) and nonvoiding detrusor contractions of rats with bladder outlet obstruction (BOO) and decrease TRPV1 expression in the bladder, resulting in improved bladder functions [10]. We also confirmed that SQW can modulate the release of ATP and expression of P2X3in trpv1 gene knockout mice [11]. In the bladder, ATP is released from the urothelium directly or indirectly to elicit responses from nerve endings by P2X3 receptor signaling. The interaction between cells of the bladder has been demonstrated to enhance individual cellular responses, and Cx43 may be involved in the relevant signals [12,13]. We hypothesized that the mechanism of SQW in modulating bladder function is related to the interaction of bladder cells, and the present study has provided valuable evidence.
Recently, we have successfully re-created OAB model rats by BOO surgery. The guinea pig OAB model was used to confirm the relation of Cx43 expression in the treatment mechanism of SQW. In vivo study was carried out using the OAB model guinea pigs with or without SQW treatment, and the sham operation group was used as the control. Micturition behavior was observed via the voided stain on paper (VSOP) test. Detrusor dynamic function was evaluated by urodynamic test. In vitro organ bath was used to study bladder distension response to various compounds. In this work, we studied the mechanism of SQW in modulating cell interaction of the bladder to improve urinary bladder functioning.
Materials and Methods
Ethics statement
The study protocols followed the rules and guidelines of the Experimental Animal Center of Guangzhou University of Chinese Medicine and were approved by the Guangzhou University of Chinese Medicine Animal Care and Use Ethics Committee (No. 00066178, 2014/02/27-2014/04/03). The experiments were in accordance with the international, national, and institutional animal experiment rules. The rats were handled according to internationally accepted principles of the care and welfare of laboratory animals (E.E.C. Council Directive 86/609, O.J. no. L358, 18/12/ 86). All animals were sacrificed by anesthesia at the end of our study.
Animal grouping and BOO surgical procedures
Healthy adult guinea pigs (weighing 300-350g, female) were provided by the Medical Experimental Animal Center of Guangzhou University of Chinese Medicine. License of guinea pigs was SCXK (YUE) 2013-0020. All guinea pigs were housed under a12h light/12h dark cycle at 20 °C-24 °C, with free access to food and water for at least 1 week before the experiments.
The BOO surgery procedures for guinea pigs were in accordance to the previously described method. Briefly, guinea pigs were anesthetized with intraperitoneal administration of 3mL/kg pentobarbital. The bladder and proximal urethra were exposed by using a lower abdominal midline incision. A 2-0 silk ligature was placed around the urethra and tied in the presence of an intraluminally placed indwelling polyethylene cannula with an outer diameter of 1mm. The abdominal wall was sutured after the polyethylene cannula was removed. Antibiotic medication (penicillin-G 400,000I.U./ kg) was used to prevent infection of the surgical area. The sham operation group was subjected to the same procedures, except that the urethra was not tied. Thirty BOO guinea pigs were grouped into the OAB model group and SQW-treated group (15 guinea pigs each, with SQW at 1170mg/kg/day). All treatments were with intragastricgated administration started at 2 days after surgery and last for 2 weeks.
Drug preparation
In our study, SQW was purchased from Hunan Hansen Pharmaceutical Co., Ltd. The process and production were similar to that of the previous report [10,11]. Briefly, all these three components (Alpinia oxyphylla Miq, Dioscorea rhizome Thunb and Aconiti tuber) were weighed at a ratio of 1:1:1 and mixed well after grinding into powder and then faint pilule. Assurance of quality control for SQW was validated according to the Chinese Pharmacopeia (Chinese Pharmacopoeia Commission), and lindane was the recorded reference standard of SQW. High-performance liquid chromatography and thin-layer chromatography were used to test the typical chemical constituents of SQW in our present experiment [14].
VSOP Analysis
All animals were individually placed in 0 with free access to food and water. They were allowed to adapt to the new environment for 24h. Urine output within 3h was measured by evaluating the surface area of the stains on the paper used for VSOP analysis (Whatman). The collected papers were imaged under ultraviolet light to visualize the urine area and analyzed using the edgedetection function of ImageJ software to determine the surface area of the individually voided urine spots. The voiding volumes of each guinea pig were calculated based on a calibration curve of surface area versus fluid drops of known volume according to our previous study [11].
Urodynamic test
Urodynamic evaluation was performed with a urodynamic measuring device (Laborie Delphis 94-R01-BT, Canada). Guinea pigs were anesthetized using 10% urethane (4.0mg/kg). Polyethylene tubing with an outer diameter of 0.9mm was inserted into the bladder through the urethra. The tubing was connected to a pressure transducer and a Harvard syringe pump using a threeway stopcock to record the intravesical pressure, and saline was infused into the bladder. Cystometrography was performed with saline infusion at 200μL/min. Maximum voiding pressure (MVP) and BP were then measured. Postvoiding residual volume (PVR) was measured by withdrawing the intravesical fluid through the catheter. Maximum bladder capacity (MBC) was calculated as infusion speed multiplied by time. Voiding efficiency (VE) was calculated as (MBC-PVR)/MBC×100% [15]. The values of individual guinea pig represent the means of two or three voiding cycles.
In vitro experimental protocols
Guinea pigs were anesthetized with urethane (Halocarbon Laboratories, USA), and the urinary bladder was quickly removed at the level of the bladder neck. The bladder body was cut open vertically and divided into strips with identical length (2mm×10mm). A strip was mounted longitudinally on a pressure transducer connected online to a Power Lab 4/30 Data Acquisition System (LABCHART 5) and to a computer with a Pentium dualcore processor for real-time monitoring of physiological force after weighing. The strips were equilibrated for at least 1h in Krebs- Henseleit (Krebs) solution (composition: NaCl 110mM, KCl 4.8mM, CaCl2 2.5mM, MgSO4 1.2mM, KH2PO4 1.2mM, NaHCO3 25mM, and dextrose 11mM) at 37 °C with the continuous bubbling of 95% O2 and 5% CO2. The strips were continuously adjusted to 1.0g resting tension.
In this experiment, bladder strips were initially treated with α, β-me ATP (100μM). Then, contractile tone was measured following the cumulative application of carbachol (10−8, 3×10−8, 10−7, 3×10−7, 10−6, 3×10−6, 10−5M), and KCl (100mM). After the contractile response to each compound reached plateau, the strip was washed thrice and allowed to equilibrate further for 30min to recover before the next compounds were added.
Real-time polymerase chain reaction (RT-PCR)
RT-PCR procedures were performed as previously described [10]. Total RNA from the bladder tissue was isolated with a TRIzol reagent and reverse-transcribed into cDNA using a RT-PCR kit (Thermo Fisher Scientific, USA). The synthesized cDNA was amplified with quantitative RT-PCR on an ABI Prism 7500 system using SYBR Green RT-PCR master mix reagent (Thermo Fisher Scientific, USA). Data were collected and analyzed using complementary computer software. Gene expression was calculated using the 2−ΔΔCt method and normalized to GAPDH expression of each sample. The expected RT-PCR product sizes and primers used in this study are as follows:
A. GAPDH: forward primers 5′-GGTGAAGGTCGGTGTGAACG-3′,
B. Reverse primers 5′-CTCGCTCCTGGAAGATGGTG-3′,
C. Cx43: forward primers 5′-TACTTCAACGGCTGCTCCTC-3′,
D. Reverse primers 5′-GGTTCTGCTCCGCACTGTAG-3′.
Western blot
Western blot was performed as previously described [10]. Tissue was homogenized, and total proteins were extracted using a total protein extraction reagent kit. Protein concentration was measured using Pierce BCA protein assay kit (Thermo Fisher Scientific, USA). Protein samples were separated on SDS-PAGE gels and transferred to PVDF membranes using a trans-blotting apparatus (Bio-Rad Laboratories, USA). The membranes were blocked with nonfat milk and subsequently incubated with the primary antibody of Cx43 (1:1000, Abcam plc, UK, the goat polyclonal antibody). After washing, the membranes were probed into antirabbit secondary antibody (1:1500, Abcam). Protein bands were visualized using ECL reagents (Bio-Rad Laboratories, USA). The intensity of target protein band was analyzed and expressed relative to β-actin density.
Data Analyses
Data are expressed as mean±standard error of the mean. For multiple comparisons, repeated-measure ANOVA (Holm–Sidak) was used. Pairwise and non-pairwise comparisons were performed via Student’s t-test. Linear regression analyses were also conducted where appropriate, and ANOVA was used to compare regression slopes and intercepts. These calculation processes were performed using SPSS 13.0 based on the number of individuals. P< 0.05 was considered statistically significant.
Result
Figure 1:Micturition times within 3h in VSOP test of the sham group, OAB model group, and SQW-treated groups are shown in the figure. The values are expressed as mean in VSOP test of the sham group, OAB model group, SQW-treated groups, and sham group. #=P< 0.05 for comparisons between the SQW-treated group and OAB model group.
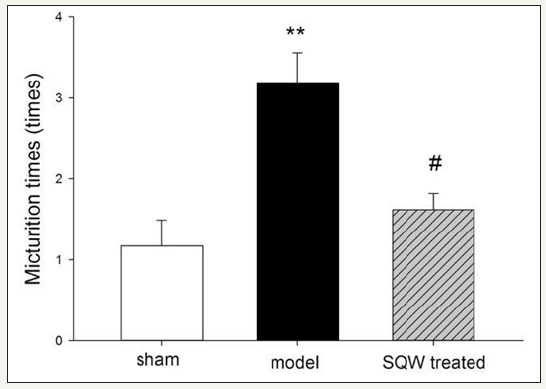
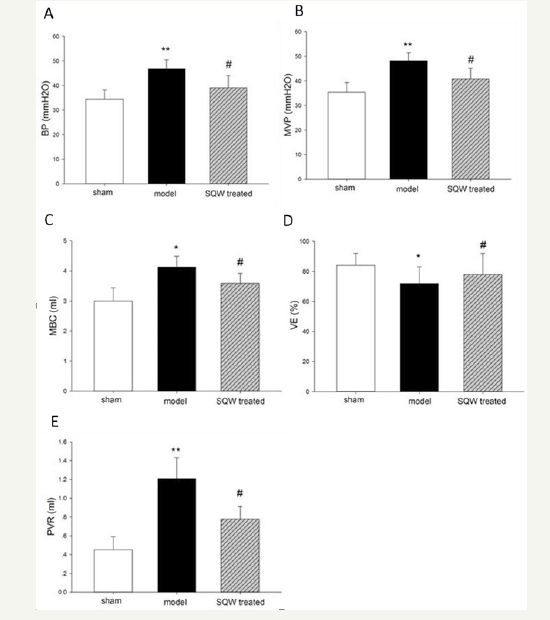
Micturition behavior of guinea pig in different groups was observed by VSOP test for 3h, and the result is shown in (Figure 1). Guinea pigs with BOO exhibited a significant increase in micturition times, whereas with SQW treatment, the micturition times of OAB model guinea pig were decreased. In accordance with previous experiments, the urodynamic results of the OAB model groups showed a significant increase in MVP and BP and bladder capacity with declining VE and increasing PVR compared with the sham group. SQW treatment was beneficial to the OAB guinea pig model, which exhibited a significant decrease in BP and reduced MBC, PVR, and improved VE (Figure 2).
Figure 2:Urodynamic parameters of the sham group, OAB model group, and SQW-treated groups are displayed in the figure. A-Bladder pressure (BP), B-Maximum voiding pressure (MVP), C-Maximum bladder capacity (MBC), D-Voiding efficiency (VE), and E-Postvoiding residual (PVR). Values are expressed as mean±standard error of the mean (SEM). *=P< 0.05, **=P< 0.01 for comparisons between the OAB model group and sham group. #=P< 0.05 for comparisons between the SQW-treated group and OAB model group.
After urodynamic tests were conducted, the bladder weight and body weight of guinea pigs were measured, and the bladder weight to body weight ratio was calculated. The bladder weight to body weight ratio of OAB model guinea pigs significantly increased. Drug administration significantly reduced these indices in SQW treatment (Table 1).
Table 1:The ratio of bladder weight to body weight.
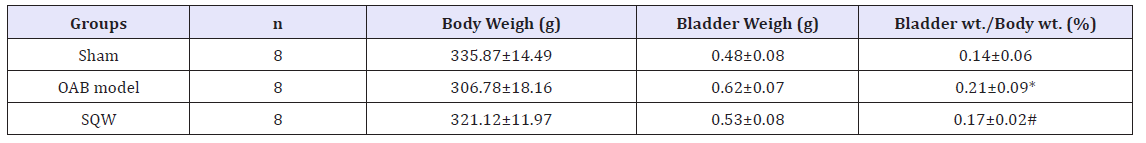
Values are expressed as mean o body Student’s t-tests or Mann-Whitney U-tests when data were not normally distributed for comparisons between OAB model group and sham groups. Paired t-test: *P< 0.05; comparisons between SQW Wan treated group and OAB model groups Paired t-test: #P< 0.05.
In vitro study was used to evaluate force response of bladder smooth muscle strips of guinea pig with BOO to α, β-me ATP (100μM), KCl (100 mM), and carbachol (10−8,3×10−8, 10−7, 3×10−7, 10−6, 3×10−6, 10−5M). The results indicated that force response of bladder smooth muscle strips of the model group was significantly increased, reducing the contraction of the bladder strips of the SQW-treated group (Figure 3).
Figure 3:Comparison of bladder strips from different groups in response to α, β-me-ATP and KCl and the concentration–response curves (CRCs) of carbachol. A: Force response to α, β-me-ATP of the sham group, OAB model group, and SQW-treated groups. B: Force response to KCl of the sham group, OAB model group, and SQW-treated groups. C: CRCs of carbachol of the sham group, OAB model group, and SQW-treated groups. Values are expressed as mean±SEM. *=P< 0.05 for comparisons between the OAB model group and sham group. #=P< 0.05 for comparisons between the SQW-treated group and OAB model group.
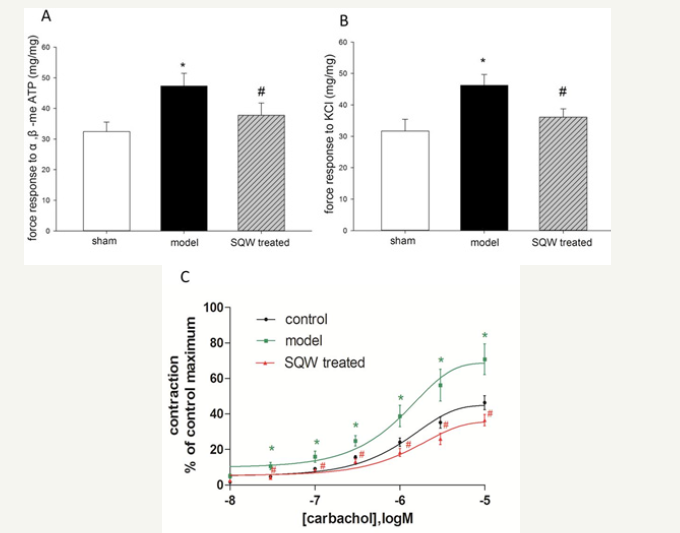
Figure 4:Cx43 mRNA and protein expression on the bladder of the sham group, OAB model group, and SQW-treated groups are illustrated in the figure. A-Cx43 protein expression of each group, B-Data from A were statistically analyzed, C-Cx43 mRNA expression of each group. Values are expressed as means of the sham group, OAB model group, and SQW-treated groups vs. the sham group. #=P< 0.05 for comparisons between the SQW-treated group and OAB model group.
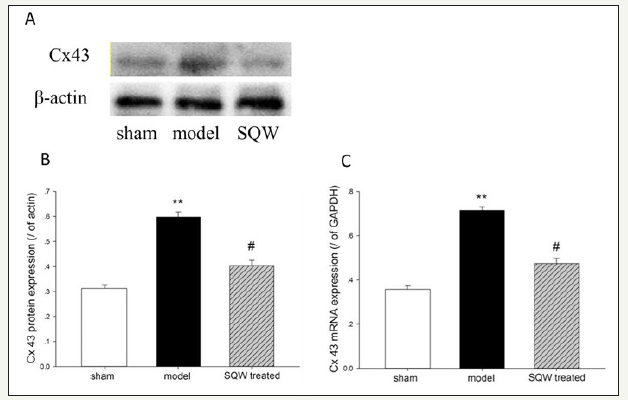
With regard to Cx43 expression, RT-PCR and Western blot demonstrated that the OAB model increased the expression of Cx43 mRNA and proteins in the bladder compared with the sham group (P< 0.01). Moreover, SQW treatment significantly reduced the expression of Cx43 mRNA and proteins in the OAB model (Figure 4).
Discussion
Outlet obstruction is a common clinical disorder, which results in smooth muscle cell rearrangement in the bladder wall and function alterations. However, the mechanisms underlying the changes of these cells remain unclear. BOO in experimental model rats is known to progress and lead to increased bladder weight and body weight loss with a hypotensive bladder [10,16]. In our present study, different to the last study reported by [10] with the OAB rat model, we established the guinea pig model by BOO. Urodynamic tests indicated that OAB guinea pigs exhibited increased MVP and BP but low VE. With the loss of bladder function, an increase in PVR was observed, which would lead to bladder filling shift at an earlier time. Micturition behavior of OAB guinea pigs measured by VSOP test showed increased micturition time compared with the sham group as a result of increasing PVR. With this pathology, the guinea pigs exhibited decreased body weight and bladder hypertrophy
The bladder is a sparsely innervated and electrically quiescent tissue, where the intercellular signaling may be particularly dependent on gap junctional communication. Intercellular communication is likely to guarantee a coordinated response of the cells forming the detrusor muscle to establish normal bladder functions [17,18]. Gap junctions are composed of intercellular channels that allow passage of small ions and molecules of neighboring cells, including ATP and cyclic adenosine monophosphate. The channels involved are mostly formed by the connexin protein family, wherein member Cx43 has been found to be the dominant isoform in the working myocardium. [19,20] were the first to report that the C-terminal tail of Cx43 interacts directly with tubulin and that microtubules preferentially terminate in areas of cell-to-cell contact. However, the exact location of Cx43 microtubule interaction is unknown. Cell culture study found that in guinea pigs and humans, the smooth muscle cells of the detrusor muscle are coupled by gap junction channels and contain Cx43 [21,22]. In the present study, Cx43 expression was detected at the mRNA and protein levels in guinea pig bladder, and BOO guinea pigs had high Cx43 expression level.
OAB caused by other pathologies should be treated immediately and appropriately. The use of medicinal herbs has increased as part of complementary and alternative medicine [23]. SQW has been used to treat lower urinary tract symptoms for decades and has shown to improve bladder function and quality of life of OAB patients [24]. It works by regulating the expression level of TRPV1 and P2X3 and modulating ATP release in the bladder. The mechanism of ion channels and neurotransmitters in normal and altered bladder function is complicated. Cx43 was believed to be involved in signals relevant to the intercellular communication between bladder cells and innervated nerves [12]. We put forward this study to further investigate the mechanism of SQW on bladder function. Similar to our latest study, SQW reversed the hypotensive situation of the bladder in OAB guinea pigs and reduced the excitation of bladder strip response to α, β-me ATP, KCl, and carbachol. These compounds can elicit normal smooth muscle excitation. Another study elucidated that the propagation of spontaneous excitation and intracellular calcium waves between smooth muscle cells is through gap junctions. These processes at least can be seen in the guinea pig bladder [25]. In our study, guinea pigs with BOO presented high expression levels of Cx43 but reduced expression was found in the SQW-treated group with good micturition behavior and urodynamic outcomes [26].
Together with the results of the in vivo study of guinea pig model with BOO and In vitro study with bladder strips, we speculated that SQW can improve OAB by modulating the effect of Cx43 in the bladder. Even though the results provided are limited and the mechanism by which SQW modulates Cx43 signaling is still unclear, this study provided valuable insights [27]. Further research is needed to uncover the mechanism by which SQW works in gap junction channels to adjust the intercellular communication of bladder cells and the active compounds of SQW [28].
Acknowledgment
This work was funded by the Specialized Research Fund for the Doctoral Program of Higher Education (no. 20124425110006), entitled “Investigating the mechanism of combination treatment of Suo Quan Wan and Shenqi Wan on OAB guinea pig through nerve-ICC-smooth muscle network”; the National Natural Science Foundation of China (no. 81673676), entitled “Effect and Mechanism of SQW on Neurotransmission Abnormality in the Treatment Recovery of Diabetic Cystopathy”; and the Science Program for Overseas Scholar of Guangzhou University of Chinese Medicine (Torch Program, no. XH20140111).
Consent for Publication
All authors have consented for publication.
Availability of Data and Materials
The raw data of our study have not been deposited in the repository, but the materials and data of our study are available to other researchers upon request. Moreover, researchers can also email us for more details about our study.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, et al. (2002) The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of the international continence society. Urology 61(6): 37-49.
- Fry CH, Sui GP, Severs NJ (2004) Spontaneous activity and electrical coupling in human detrusor smooth muscle: implications for detrusor overactivity? Urology 63(3 Suppl 1): 3-10.
- Véronique P, Behr RD, Oger RS, Rouprêt M, Chartier KE (2013) Involvement of connexins 43 and 45 in functional mechanism of human detrusor overactivity in neurogenic bladder. Urology 81(5): 1108(e1- e6).
- John H, Walch M, Lehmann T. Maake C (2009) Connexin 45 expression in the human obstructed detrusor muscle. World J Urol 27(3): 411-418.
- Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, et al. (2007) Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293(4): 1018-1025.
- Haferkamp A, Mundhenk, Bastian P, Reitz A, M ller, et al. (2004) Increased expression of connexin 43 in the overactive neurogenic detrusor. Eur Urol 46(6):799-805.
- Oshiro T, Miyazato M, Saito S (2014) Relationship between connexin43- derived gap junction proteins in the bladder and age- related detrusor underactivity in rats. Life Sci 116(1): 37-42.
- Cao HY, Wu QH, Huang P, He JY (2009) Impacts of the formula of Suoquanwan (SQW) on expression of AQP-2 mRNA and AVPR-V2 mRNA in the kidney of rat polyuria model of Yang-deficiency. Zhong Yao Cai 32(6): 926-928.
- Li SW, Hu LJ, Wu QH, Huang P, Cao HY (2011) The influence of suoquan capsule on the mRNA expression of CYP11B2 in deficiency of the kidney and diuresis rats. Zhong Yao Cai 34(1): 80-83.
- Lai H, Tan B, Lian Z, Yan Q, Lian Q, et al. (2015) Effect of the Chinese traditional prescription Suo Quan Wan on TRPV1 expression in the bladder of rats with bladder outlet obstruction. BMC Complement Altern Med 15: 424.
- Lai H, Yan Q, Cao H, Chen P, Xu Y (2016) Effect of SQW on the bladder function of mice lacking TRPV1. BMC Complement Altern Med 16(1): 465.
- Fry CH, Sui GP, Kanai AJ (2007) The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn 26(6 Suppl): 914-919.
- McCloskey KD (2010) Interstitial cells in the urinary bladder-localization and function. Neurourol Urodyn 29(1): 82-87.
- Liang Zhijian, Jiang Weiwen, CAO Hongying (2015) Study on quality standard of Suoquan Pills. Traditional Chinese Drug Research & Clinical Pharmacology 26(2): 236-237.
- Choi BH, Jin LH, Kim KH, Kang SA, Kang JH, et al. (2013) Cystometric parameters and the activity of signaling proteins in association with the compensation or decompensation of bladder function in an animal experimental model of partial bladder outlet obstruction. Int J Mol Med 32(6): 1435-1441.
- Haefligera JA, Pascal N, Paolo M (2004) Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62(2): 345-356.
- Haefliger JA, Tissieres P, Tawadros T, Formenton A, Bény JL, et l. (2002) Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res. 274(2): 216-225.
- Christ GJ, Day NS, Day M, Zhao W, Persson K, et al. (2003) Increased connexin 43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol 284(5): R1241-1248.
- Stroemlund LW, Jensen CF, Qvortrup K, Delmar M, Nielsen MS (2015) Gap junctions-guards of excitability. Biochemical Society Transactions 43(3): 508-512.
- Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, et al. (2001) Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11(17): 1364-1368.
- Neuhaus J, Wolburg H, Hermsdorf T (2002) Detrusor smooth muscle cells of the guinea-pig are functionally coupled via gap junctions in situ and in cell culture. Cell Tissue Res 309(2): 301-311.
- Neuhaus J, Weimann A, Stolzenburg JU, Wolburg H, Horn LC, et al. (2002) Smooth muscle cells from human urinary bladder express connexin 43 in vivo and In vitro. World J Urol 20(4): 250-254.
- Charrua A, Cruz CD, Narayanan S, Gharat L, Gullapalli S, et al. (2009) GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol 181(1): 379-386.
- Andersson KE (2007) LUTS treatment: future treatment options. Neurourol Urodyn 26(6 Suppl): 934-947.
- Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H (2001) Origin and propagation of spontaneous excitation in smooth muscle of the guineapig urinary bladder. J Physiol 530(Pt 2): 273-286.
- Chughtai B, Elizabeth K, Richard L, Alexis T, Franklin L, et al. (2013) Use of herbal supplements for overactive bladder. Rev Urol 15(3): 93-96.
- Choi BH, Jin LH, Kim KH, Kang SA, Kang JH, et al. (2013) Cystometric parameters and the activity of signaling proteins in association with the compensation or decompensation of bladder function in an animal experimental model of partial bladder outlet obstruction. Int J Mol Med 32(6): 1435-1441.
- Chinese Pharmacopoeia Commission. China Pharmacopoeia Committee. Vol. 1218.
© 2019 Hongying Cao. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






