- Submissions

Full Text
Advances in Complementary & Alternative medicine
Heme and HIV: Complementary Therapies for the Prevention and Treatment of HIV through Mitochondrial Pathways
Avrille Semo1, Nicholas A Kerna1,2* and Orien L Tulp1
1College of Medicine, USA
2Suriwongse Medical Center, Thailand
*Corresponding author: Nicholas A Kerna, College of Medicine, 4288 Youngfield Street, Wheat Ridge, CO 80033, USAa
Submission: January 19, 2019;Published: January 23, 2019

ISSN: 2637-7802 Volume3 Issue5
Abstract
Heme formation can be described and understood via various entrees. Herein, a foundation of heme formation will be characterized by four distinct, but interconnected, tracts: locations of heme formation, pathways of heme formation, functions of heme, and disorders in heme formation. This paper reviews the healthy state of mitochondria and the cell (and, thus normal heme formation and function) in unperturbed states and correlates disruptions in one or more of the four tracts mentioned above regarding heme in disease, in particular, HIV. This investigation (on a basic, fundamental, and condensed level) lays the groundwork for assessing certain conditions (and risk factors thereof) from a cellular level by connecting defects in heme formation and mitochondrial aberrations with complementary therapies for the prevention and treatment of HIV.
Keywords: Anemia; Delta-aminolevulinic acid; Cytochrome P450; Enzyme defect; Heme; HIV; Krebs cycle; Mitochondria; Porphyria; Prosthetic groups; Protoporphyrinogen; RBC
Abbreviations: ALA: Aminolevulinic Acid; ALA: Dehydrogenase; ΔALAS2: Delta-Aminolevulinate Synthase 2; CAC: Citric Acid Cycle; ΔALA: Delta- Aminolevulinic Acid; HIV: Human Immunodeficiency Virus; MRC: Mitochondrial Respiratory Chain; PBMC: Peripheral Blood Mononuclear Cell; PCG-1a: Peroxisome Proliferator-Activated Receptor Gamma Coactivator; RBC: Red Blood Cell
Introduction
Why do certain people exposed to HIV contract the disease while others do not? This medical “conundrum” is due to many factors. These factors can be categorized as exogenous and endogenous.
Exogenous factors in contracting HIV
Viral load: If the viral load of the HIV positive person is low, then the chance of contracting HIV is relatively low. If the viral load is high, the chance of contracting HIV is higher. (Also considered are carrier viral factors that can escape the natural immune response of the intended host.)
Exposure rate; single exposure versus multiple exposures: If a person is exposed to a low viral load, but on multiple occasions, their risk for contracting HIV increases over single exposure or less repeated exposure to a low viral dose [1].
Stress (physical, emotional, environmental): Prolonged stress diminishes the immune response and the effectiveness of the immune system.
Diet: A poor diet or a diet that subverts the mitochondria can weaken cellular response leaving a person more susceptible to HIV than they otherwise would have been [2]. (This mitochondrial connection will be considered in the following Discussion section.)
Endogenous factors in contracting HIV
Compromised host: Concurrent infections or diseases can leave a person most susceptible to contracting HIV in exposure [1]. (As above, also considered are carrier viral factors that can escape the natural immune response of the intended host.)
Host cellular factors: The proper modulation of the innate or acquired immune responses to infection is a determinant; this modulation depends on host cellular factors.
Enzymatic disorders: Certain enzymes are fundamental in the pathway of heme biosynthesis; disorders in these enzymes or their utilization can disrupt heme formation and leave the host susceptible for attack by HIV at those associated sites where heme is disrupted [3].
Genetic defects: Defects in any gene involved in the synthesis of precursors of heme formation can also disrupt heme formation and leave the host susceptible for attack by HIV at those associated sites where heme is disrupted [4].
Note: A small percentage of the population (estimated at less than 1% in the UK) have genetic protection against HIV [5].
The following discussion looks at the above factors through pathways of heme formation in regard to HIV prevention and treatment with the use of complementary therapies.
Discussion
Foundation in heme formation: Locations
Heme formation takes place in the mitochondria and cytoplasm. Heme formation occurs in every cell of the body, except red blood cells (RBCs). RBCs have no mitochondria; therefore, they cannot be a location for heme formation.
Foundation in heme formation: Pathways
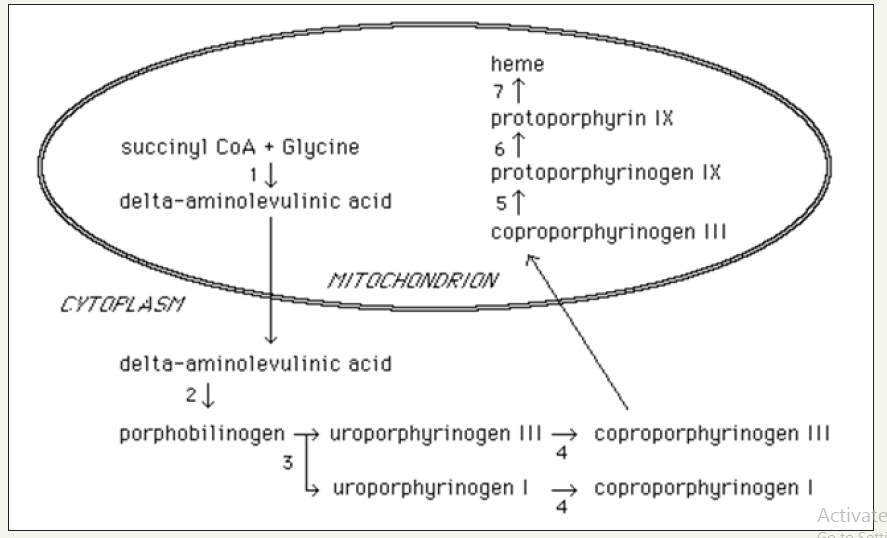
The highlights of the heme formation are described below. See Figure 1 for a rendering of the essential steps of heme formation.
A. Delta-aminolevulinic acid (dALA) initiates the heme formation pathway. dALA is a product of the citric acid cycle (CAC) [aka, tricarboxylic acid cycle (TCA) or Krebs cycle] from the reactants amino acid glycine with the rate-limiting enzyme ALA synthase within the mitochondria.
B. The pathway of heme formation continues in the cytoplasm. It is no longer the enzyme ALA synthase, but ALA dehydrogenase that moves the reaction forward, and begins to produce intermediates.
C. Intermediates are produced during the enzymatic process known as porphyrin synthesis. This cytoplasmic process ends when the enzyme Cp-III oxidase in the cytosol carries the process back into the mitochondria, resulting in the synthesis of protoporphyrinogen III.
D. The next enzymatic process in the pathway of heme formation is the production of protoporphyrin IX which is promoted by the enzyme protoporphyrin IX in the mitochondria.
E. Next, Fe2+ and the enzyme ferrochelatase participate in the enzymatic process with protoporphyrin to form heme in the mitochondria.
F. After this final step, heme formation is complete [6] (Figure 1).
Figure 1:The pathway of heme formation as depicted in University of Utah Medical School Library online resources [6].
Foundation in heme formation: Homeostatic functions
a. Heme acts as an intermediate molecule in the catabolic reaction of hemoglobin,
b. Heme molecules play a central role in the human body that includes not only electron transfer during respiration but also electron transfer during enzyme catalysis in human body cells.
c. Heme-containing-cytochromes are integral components of various respiratory electron transport chains.
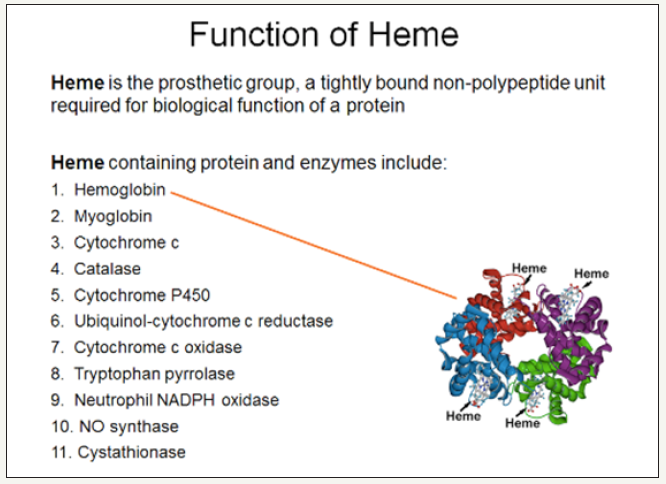
d. Heme serves as a prosthetic group [7]. Prosthetic groups include “hemoglobin, myoglobin, catalases, peroxidases, and cytochromes P450”. Specifically, heme functions as a prosthetic group “in sensor proteins for diatomic gases such as 02 or NO” [8] (Figure 2).
Figure 2:The function and structure of nonprotein heme [9].
Disorders in heme formation due to enzyme irregularities
An essential function of heme is in its role as an intermediate molecule in the catabolic reaction of hemoglobin. Therefore, if one of the enzymes in heme formation is altered, due to a defect in the enzyme, the normal heme in the biochemical pathway of catabolism of hemoglobin is disrupted.
Enzymatic disruption or malfunction can lead to a class of disorders termed, porphyria. Heinz et al. [7] state that “in humans [disruptions of] the enzymes that participate in the many steps of the heme pathway can be responsible for disorders, such as porphyrias and X-linked sideroblastic anemia”.
Over thirty years ago, Bloomer [3] connected many disorders of porphyrias with certain enzyme defects as listed below.
I. Disorder: acute intermittent porphyria; enzyme defect: uroporphyrinogen I synthase.
II. Disorder: protoporphyrin; enzyme defect: heme synthase.
III. Disorder: hereditary coproporphyria; enzyme defect: coproporphyrinogen oxidase.
IV. Disorder: porphyria cutanea tarde; familial enzyme defect: uroporphyrinogen decarboxylase.
V. Disorder: porphyria cutanea tarde; sporadic enzyme defect: uroporphyrinogen decarboxylase.
VI. Disorder: congenital erythropoietic; enzyme defect: uroporphyrinogen III synthase autosomal recessive metabolic disorder.
VII. Disorder: congenital erythropoietic; enzyme defect: cosynthase/uroporphyrinogen autosomal recessive genetic disorder.
At that time, Bloomer [3] determined that the relationship of many disorders of porphyria and the responsible enzyme defect were clear (as listed above); however, others were unclear. The following are examples of certain porphyria disorders, the causes of which, at that time, were equivocal.
1) Disorder: variegate porphyria: Enzyme defect heme synthase (later determined to result from the deficient function of the enzyme uroporphyrinogen III synthase).
2) Disorder Variegate porphyria: Enzyme defect protoporphyrinogen oxidase (later confirmed).
3) Disorder: unnamed; enzyme defect: ALA dehydrase (later determined, and named, delta-aminolevulinic acid (ALA) dehydratase(ALAD) porphyria (ADP) which results from ALAD deficiency due to a genetic defect) [8].
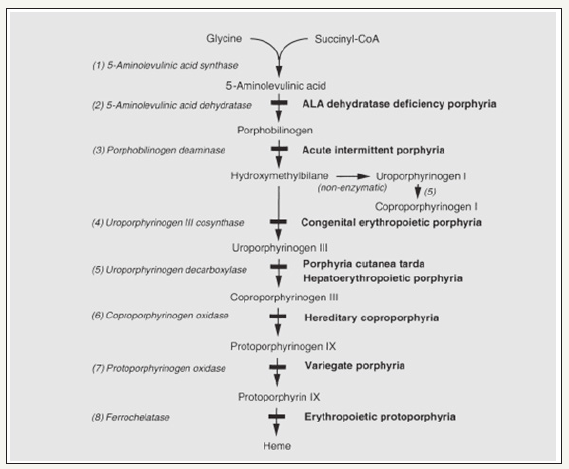
According to Casademont et al. [9], a further connection has been established between many disorders of porphyrias and the following defect: acute porphyria associated not only with mtDNA depletion but also with great MRC disturbances and increased oxidative damage (Figure 3).
Disorders in heme formation due to gene defects
In essence, most enzyme disorders are genetic disorders. Enzyme disorders are expressed, principally, by genetic defects: defects in a gene or that codes for the synthesis of certain enzymes or precursors. The pathway of X-linked sideroblastic anemia is similar to the defects in porphyrias that are enzyme-related. X-linked sideroblastic anemia is a result of a defect in the ALAS2 gene that encodes delta-aminolevulinate synthase 2 (or ALAS2 ) proteins and occurs in the first step of heme biosynthesis [10,11].
Figure 3:Egger, Lee, and Anderson (2008) illustrated the heme biosynthesis, intermediates and enzymes, defects and resultant porphyrias [11].
Note: The porphyrias caused by certain enzyme defects are listed in bold.
Disorders in heme formation due to substitution reactions
Enzyme defects do not solely cause disorders in heme formation. When another functional group replaces one functional group in a chemical compound, it is considered a substitution reaction. Specifically, a disorder is manifest by the production of hemin that takes place as Fe3+ is utilized instead of Fe2+. This result is an adverse substitution reaction [10].
It has been put forth that HIV results as an adverse substitution reaction. HIV fervently attacks red blood cells. Heme formation does not take place due to HIV “substitution”. Conversely, Schmidt et al. [11] in vitro experiments demonstrated that heme oxygenase-1 and its oxidative products and related compounds of the heme oxygenase system inhibit replication of human immunodeficiency virus.
HIV-Mitochondria Connection
Casademont et al. [9] demonstrated the vast impact of HIV disturbance on different components of the mitochondria in the human cell with the goal “to investigate the effects of HIV infection on mitochondrial DNA (mtDNA) content and other mitochondrial parameters.
They went on to state: We used peripheral blood mononuclear cells (PBMCs) from 25 asymptomatic antiretroviral-naïve human immune-deficiency virus (HIV)-infected patients and from 25 healthy control subjects. The HIV-infected patients had significant decreases in mtDNA content (decrease, 23%; P< .05) and in the activities of mitochondrial respiratory chain (MRC) complex II (decrease, 41%; P< .001), MRC complex III (decrease, 38%; P< .001), MRC complex IV (decrease, 19%; P=.001), and glycerol-3- phosphate dehydrogenase (decrease, 22%; P & lt; .001), along with increased lipid peroxidation of peripheral blood mononuclear cell (PBMC) membranes (P=.007) [9].
Based on the symptom of weakness in many HIV patients (young and old), a hypothesis for this common phenomenon is that HIV may not only drain the body of energy reserves but also may block or prohibit energy production in the body. Therefore, HIV infection is associated with mtDNA depletion, great MRC disturbances, and increased oxidative damage [10].
Innate mitochondrial resistance to HIV
In those people that remain HIV negative, even after exposure to HIV, it can be posited that mitochondria are more “powerful” in their immunologic response due to mechanisms of action, support systems, products and by-products, and pathways that results in exposed persons remaining HIV negative. Mitochondria may have a mechanism by which they can “supercharge” in order to thwart the full effect of the HIV. Peroxisome proliferator-activated receptor gamma coactivator (PGC-1a) may be a key component in diminishing the full effect of HIV as described by Ventura et al. [4] in Transcriptional control of mitochondrial biogenesis: the central role of PGC-1a: “Recent advances in molecular biology have started to elucidate the transcriptional events governing mitochondrial biogenesis. In particular, a great step was taken with the discovery that PGC-1a is the master regulator of mitochondrial biogenesis” [4].
Therefore, in the mitochondria, when HIV is recognized, a formidable reproductive, regenerative, and genetic signaling response is generated that decodes possible substitution sites for HIV reducing the odds of HIV replication and substitution sequences needed for HIV infiltration into the human body. This signal failure may prevent HIV transmission as HIV cannot maneuver to substitute to encode, nor can it replicate. Thus, when stagnant, the HIV may become deoxygenated by-products of heme formation [4]. In the most simplistic terms, the HIV is surrounded, it cannot “breathe”, it cannot communicate or signal to substitute or replicate, it is weakened, and it then “suffocates” and dies.
Pharmaceutical interventions for HIV and aberrations in heme biosynthesis
It is beyond the scope of this article to list the pharmaceutical interventions, their side effects, and the pros and cons thereof; there are numerous resources relating such. The focus of this paper is on complementary therapies.
Complementary therapies for the prevention and treatment of HIV: Mitochondrial can be regulated to increase energy in the cells; what, if anything, can the healthcare provider and the HIV positive patient focus on in order to facilitate the regulatory process of mitochondrial biogenesis?
Diet: Diet considerations are also important parts of treatment plans because certain foods can be correlated to porphyria disorders [2]. In particular, one diet choice that is important to discuss is the value of implementing a ketogenic diet that has a benefit to mitochondria of sparing it for other vital jobs since “the body starts using fats as a primary energy source of energy instead of carbohydrates” [12].
Dietary supplements: CoQ10 supplements can help improve not only the function of mitochondria by insulating it from oxidative damage from free radicals but also it can improve the lifespan of the mitochondria [12]. Consuming diets high in protein and low in carbohydrates has a positive effect on the mitochondria. These dietary components increase mitochondrial oxidation which extends the life of mitochondria.
Physical therapy: Further, the importance of getting a massage and, according to Hemal [12] the “removal of toxins, through deep tissue massages results in better blood and oxygen circulation. A deep tissue massage also leads to mitochondrial biogenesis, an important process that involves the growth and division of pre-existing mitochondria”. These actions promote healthy gastrointestinal and musculoskeletal system functioning.
Exercise: Hemal [12] reported: “by getting a healthy workout in, you enhance the functioning of existing mitochondria on your body and facilitate the production of new ones in a phenomenon called the mitochondrial biogenesis”.
It is elemental to remain judicious with exercise regimens as with all lifestyle changes [2]. Hemal [12] also reported: “all forms of exercise are beneficial for mitochondria; however, Dudley, Tullson, & Terjung [13] studied the correlation between the intensity of exercise on mitochondria. According to the study, “extending a workout from 30 to 60 minutes leads to a better mitochondrial function. However, extending it [exercise] from 60 to 90 minutes has shown no improved effect on mitochondria”.
Supplementation for Related Symptoms
There are numerous psychological cofactors to consider for the HIV patient [14]. Psychological and physical stressors can result in insomnia and irregular sleep patterns. Pharmacological sleep aids can have adverse effects on certain individuals. Hemal [12] opined: “liposomal melatonin is known to help you sleep, but it also has a positive effect on mitochondria and works well as an antioxidant. Melatonin has physiological effects on normal mitochondria, such as prevention of mitochondrial impairment, energy failure, and apoptosis in oxidatively damaged mitochondria”.
Conclusion
Globally, HIV is a widely prevalent disease that results in not only millions of deaths worldwide, but also affects millions of births worldwide. The mechanisms by which HIV is spread and the pathways of its replication, although better understood today than in previous decades, needs continuing research; in particular, in regard to complementary and alternative therapies to help maintain and boost mitochondrial function in the prevention and treatment of HIV. Complementary therapies, as described herein, may have positive effects, have little to no adverse effects, and have no known negative interactions with pharmaceuticals and standard treatment for HIV. Therefore, these current complementary therapies and interventions should be recognized and utilized; new applications (such as botanicals and other natural interventions) should be pursued. In high risks groups and populations, these complementary therapies could act as affordable and valuable tools, especially in public health and for countries with limited resources.
Conflict of Interest
The authors declare that this paper is written in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Fenner L, Atkinson A, Boulle A, Fox M, Prozesky H, et al. (2017) HIV viral load as an independent risk factor for tuberculosis in South Africa: collaborative analysis of cohort studies. J Int AIDS Soc 20(1): 21327.
- American Porphyria Foundation (2017) Nutrition in the acute porphyrias.
- Bloomer JR (1981) Enzyme defects in the porphyrias and their relevance to the biochemical abnormalities in these disorders. Journal of Investigative Dermatology 77(1): 102-108.
- Ventura CR, Garnier A, Veksler V (2007) Transcriptional control of mitochondrial biogenesis: The central role of PGC-1a. cardiovascular re 79(2): 208-217.
- Avert org (2018) HIV and aids in the United Kingdom (UK).
- University of Utah Medical Library. Heme synthesis.
- Layer G, Reichelt J, Jahn D, Heinz DW (2010) Structure and function of enzymes in heme biosynthesis. Protein Science 19(6): 1137-1161.
- Heme biosynthesis L5. MCPM Exam 3, Memorang.
- Casademont J, Cardellach F, Deig E, Garrabou G, Gatell JM, et al. (2004) Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clinical Infectious Diseases 39(5): 710-716.
- Egger NG, Lee C, Anderson KE (2008) Disorders of heme biosynthesis.
- Schmidt W, Mathahs MN, Meleah M, Zhu Z (2012) Heme and HO-1 Inhibition of HCV, HBV, and HIV. Front Pharmacol 3: 129.
- Hemal V (2016) 10 ways to combat mitochondria misconduct and gain energy today. Honey Colony Inc.
- Dudley G, Tullsons P, Terjung R (1987) Influence of mitochondrial content on the sensitivity of respiratory control. The Journal of Biological Chemistry 262(19): 9109-9114.
- Kerna NA (2018) A Public Health Concern: Special Considerations for the HIV Patient Psychological Cofactors to Consider. SM Prev Med Public Health 2(1): 1014.
© 2019 Nicholas A Kerna. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






