- Submissions

Full Text
Advances in Complementary & Alternative medicine
Real-World Safety and Efficacy of Ribavirin – Free Regimen of Ombitasvir/Paritaprevir/ Ritonavir in HCV Dialysis Patients
Moutaz D1,2, Fadwa S3, Prem C2, Mohiuddin A1, Aliaa A4, Elsayad E5, Khater I5 and Elesnawi MA5
1Department of Gastroenterology and Hepatology, Qatar
2Department of Medicine, Qatar
3Department of Nephrology, Qatar
4Department of Pathology, Qatar
5Clinical Pharmacist, Qatar
*Corresponding author: Moutaz D, Department of Gastroenterology and Hepatology, Qatar
Submission: January 19, 2019;Published: January 28, 2019

ISSN: 2637-7802 Volume4 Issue1
Abstract
Introduction: HCV prevalence in hemodialysis patients in Qatar is 8.4% (predominantly Genotype 4). Since the launch of the Qatar plan for “HCV control by 2020”, the treatment of HCV in hemodialysis patient has been a challenge. The approval of ombitasvir, paritaprevir, and ritonavir (Viekirax) has been accepted as a treatment option in this group of patients. We aim to explore the effectiveness and safety of this regimen in a Genotype 4 predominant population without using ribavirin, known to cause anemia.
Method: Non-Interventional, single-center cohort study, including retrospective collection of real -world data on 40 hemodialysis patients infected with HCV, 19 of them completed the 12 weeks with ribavirin-free Viekirax regimen (plus dasabuvir for G1) and 12 weeks follow up period. The proportion of patients achieving SVR12 presented and the confidence intervals of SVR rates were calculated using exact binomial methods and repeated-measures analysis of variance (ANOVA) was applied to assess differences in various laboratories and biochemical parameters measured over different time points.
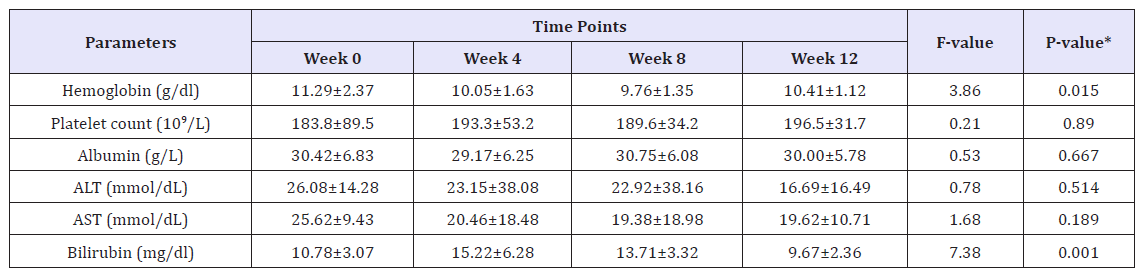
Results: The mean age of patients was 57.04±16.79 years. Most of patients had genotype 4 (n=14, 73.7%), then genotype 1 (n=3, 15.8%). One patient had genotype 1 & 4 and one had genotype 4 &6. Overall SVR 12 was 94.7% (18/19) (95% CI, 75.4 to 99.1). 26.7% were Child A cirrhosis. There was no difference in SVR 12 between low grade fibrosis (stage≤2) (14/14;100%(95%CI 78.5to100%) and highgrade fibrosis (stage≥3) (4/5; 80% (95% CI 37.6 to 94.4%); P=0.263. Repeated measure analysis of variance (ANOVA) showed mean Hb levels decreased significantly from Week 0 (mean Hb 11.3±2.4) to week 24 (mean ALT 10.4±1.1); P=0.015. Mean platelet counts showed an insignificant increasing trend from Week 0 to week 12, (P=0.890). Both mean AST and ALT levels observed to decrease with linear trend from week 0 to week 12, (P>0.05). Bilirubin showed a transient increase by 4. Regarding safety, no patient discontinued treatment because of an adverse event, 2 patient required blood transfusions.
Conclusion: Ombitasvir/Paritaprevir/Ritonavir combination therapy without Ribavirin, is effective and safe, with low rates of anemia and treatment discontinuation in patients undergoing dialysis due to ESRD.
Background
The frequency of HCV infection remains high in patients with chronic kidney disease (CKD) and plays a detrimental role in their morbidity and mortality. In Qatar, the burden of HCV infection was significantly higher in the dialysis patients compared to the general population (8.4% vs. 2%), and genotype 4 represents 65% of the infected cases on dialysis. In addition, to improve quality of life, many clinicians recommend, that HCV should be treated, in hemodialyzed (HD) patients, before their enlistment for kidney transplantation in order to avoid the reactivation of the virus after transplantation [1]. However, there is no evidence suggesting that chronic hepatitis C exhibits a more aggressive course in CKD subjects under conservative management, and fibrosis in these patients is usually mild and cirrhosis is rare [2,3]. Since the launch of the National Qatar plan for “HCV control by 2020”, the treatment of HCV in HD patients was challenging. The advent of the new directacting antivirals (DAAs), has revolutionized treatment paradigms for HCV, including HD patients.
Two oral regimens, with high safety and efficacy and ease of administration, have been recently approved for the treatment of HCV in advanced CKD: elbasvir/grazoprevir [4] and Ombitasvir/Paritaprevir/ Ritonavir/ Dasabuvir with or without ribavirin [5]. These medications are metabolized largely in the liver, with low renal clearance, thus can be used safely in patients with chronic renal failure, with new opportunities for cure in this difficult-to-treat population [6]. The patients on HD have a higher incidence of anaemia and ribavirin can accentuate treatment-related hemolytic anemia in RBV-containing DAAS regimens and put these patients at greater risk of developing severe treatment-related anemia [7]. A very good results in terms of the efficacy and safety of Ombitasvir/Paritaprevir/Ritonavir, has been established in clinical trials, however, real-world, data were lacking in HD patients, especially in HCV genotype 4, in order to confirm this profile. The aim of this study was to report our realworld experience regarding evaluation and assessing the efficacy, safety and tolerability of, Ribavirin-Free, or short course ribavirin, Ombitasvir/Paritaprevir/Ritonavir/Dasabuvir, for HCV treatment in patients on regular hemodialysis.
Methods
Non-Interventional, observational, retrospective, singlecenter cohort study, including collection of real-world data on 40 hemodialysis patients infected with HCV, followed Hamad Hospital outpatient, from January 2016 to June 2017. This study included 40 HCV patients undergoing hemodialysis. Patients with decompensated cirrhosis, whether Child B or C, were not eligible for this therapy. Administration of Ribavirin was independent of initiation of dialysis. Phosphorus-binding drugs were administered 4 hours before or after DAAs, to avoid interference with drug absorption. All patients were treated for twelve weeks with OMV/ PTV/RTV (12.5mg/75mg/50mg orally once daily), for genotype 4 patients. Dasabuvir (250mg twice daily) was added for genotype 1 patients. Ribavirin 200mg was added during the first 2 weeks of therapy, for those with hemoglobin>9gm/dL. All patients completed the 12 weeks with ribavirin-free therapy (Figure 1).
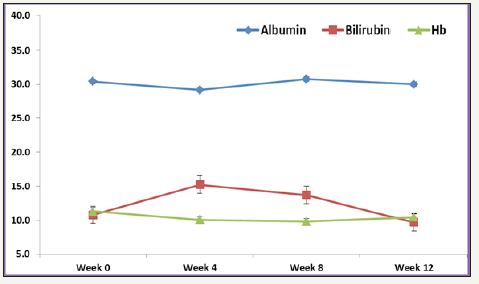
Figure 1:Mean Albumin, Bilirubin and Hb levels over different time points.
The following were initially recorded in all cases: personal data, history of comorbidities, time on renal replacement therapy with dialysis and laboratory tests (CBC, AST, ALT, Bilirubin, Albumin, Creatinine, eGFR, Alkaline phosphatase, Free T4, TSH, Cholesterol, Triglyceride and serum HCV RNA). On-treatment assessments included standard laboratory testing, serum HCV RNA, and symptom-directed physical examinations, as well as details of concomitant medication were collected before the start of therapy and at weeks 2,4,12 and 12 weeks post treatment. Adverse events (AEs) were evaluated at study visits. The primary efficacy endpoint was sustained virologic response at week 12 after the end of treatment (SVR12), analyzed by intention-to-treat (Table 1).
Statistical Analysis
The proportion of patients achieving SVR12 presented and the confidence intervals (CIs) of SVR rates were calculated using exact binomial methods. Baseline demographic, clinical, laboratory and biochemical characteristics were described with frequencies (percentage) and mean±SD or median and range as appropriate. We used the repeated-measures analysis of variance (ANOVA) to assess difference in various laboratory and biochemical parameters such as Hb, Platelet, AST, ALT, Albumin and Bilirubin over different time points (Week 0 to Week 12). Pictorial presentations of the key results were made using appropriate statistical graphs. A twosided P value< 0.05 was considered to be statistically significant. All statistical analyses were done using statistical packages SPSS 22.0 (SPSS Inc. Chicago, IL).
Result
Patients characteristics
Various laboratories and biochemical parameters over Week 0 to week 12 were compared and shown in Table 1. The mean age for studying participants was 57.04±16.79 years, and 73.7% were males. The majority of patients had genotype 4(n=14, 73.7%), followed by genotype 1 (n=3, 15.8%) and one patient had genotype 1&4 and one patient had genotype 4&6. 26.7% were Child A cirrhosis. At baseline, overall mean Hb was 11.1±2.4g/dl (range, 7-15.2g/dl), mean platelet count 175.4±82.7 10^9/L (range, 15.7- 341 10^9/L), mean ALT 35.6±34mmol/L (range, 6-127mmol/L), mean AST 33.6±20.8mmol/L (range, 14-83mmol/L), mean albumin was 31.5±6.7g/L (range, 17-42g/L) and total bilirubin (TBIL) was 11.5±3.9mg/dl (range, 5.8-21.3mg/dl) (Figure 2).
Figure 2:Mean AST and ALT levels over different time points.
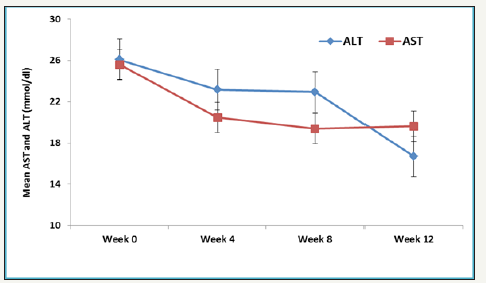
Table 1:*P-value computed using repeated measure analysis of variance (ANOVA) statistical method.
Efficacy and safety outcome measures
Overall rate of sustained virologic response (SVR 12) was 94.7% (18/19) (95% CI, 75.4 to 99.1). There was no significant difference in SVR 12 between low grade fibrosis (stage≤2) (14/14; 100% (95% CI 78.5 to 100%) and high-grade fibrosis (stage≥3) (4/5; 80% (95% CI 37.6 to 94.4%); P=0.263. The mean values of various laboratory and biochemical parameters measured were compared from baseline to week 4, week 8 and week 12. Repeated measure analysis of variance (ANOVA) showed mean Hb levels decreased significantly from Week 0 (mean Hb 11.3±2.4) to week 24 (mean ALT 10.4±1.1); P=0.015. Mean platelet showed an increasing trend from Week 0 to week 12, however, this difference didn’t reach statistical significance (P=0.890). Both mean AST and ALT levels observed to decrease with linear trend from week 0 to week 12, however, this difference was statistically insignificant (P>0.05). Bilirubin measured over different weeks showed non-liner trend and relationship with time points (increased at week 4 and 8 and then decreased at week 12). One patient who did not achieve SVR 12 was post liver transplant status.
Adverse effects
There is no adverse event-related discontinuations or dose interruptions of study medications, including ribavirin, were noted, the most serious adverse effect was, Anemia requiring blood transfusion in one patient, on ribavirin group. The most common adverse event was headache, which was reported by 29% of the patients. Transient elevation in liver enzyme elevations, within 2-3 weeks of initiation of therapy, were noticed in 2 cases (10%). There is no significant difference in tolerability among patients with compensated cirrhosis, compared to those with low grade of fibrosis. Regarding drug interaction with concomitant drugs, apart from, Statins discontinued whenever possible, no dose adjustment or medication discontinuation required for the concomitant medications, including calcium channel blockers, levothyroxine, calcium bicarbonate or other medication
Discussion
The real-life outcome of the current study re-confirm the previous clinical trial, which reported that, the overall, treatment of CHC in patients on HD is highly effective, with SVR12 rates 94.8%, similar to those seen in patients without CKD. SVR in the current study was similar to, RUBY-I clinical trial, where Pockros et al. A reported SVR12 rate of 90%, in GT1 and 97% in GT4, patient with CRK4-5 and treated with OBV/PTV/r+DSV ± RBV [5]. Genotype did not show an effect on response to treatment, suggesting that Ribavirin-free, is as effective and feasible therapy for GT4-infected individuals as OBV/PTV/r+RBV [8]. Interestingly, we observed high response rate in patients with High grade of fibrosis, providing evidence that Viekirax may be safely and effectively used in patients with HCV infection on dialysis therapy who have factors predicting an unfavorable response to treatment, such as compensated cirrhosis. Our study analyzed the changes of hemogram and hepatic function indices and the frequency of AEs associated with viekirax in hemodialysis subpopulation. The transient increase in hepatic transaminases, emphasis on early monitoring, as early as week 2 of treatment to monitor for abnormalities but highlight that AST/ ALT elevations for those without cirrhosis did not have an apparent clinical consequence, and discontinuation or modification of the dose is not required.
Hematologic adverse events, which were frequently observed among HD patients receiving ribavirin-based antiviral regimens, were rare in our study, this is probably because ribavirin has no, or minimal, role HCV positive genotype 4 patients, similar to Genotype 1b, who are treated with Viekirax [9]. The overall VIEKIRAX tolerability profile was similar in all patients regardless the stage of fibrosis.
Conclusion
The concordance with clinical trials provides reassurance that the reported efficacy of this treatment in clinical trials will translate to its use in routine clinical practice. Successful HCV antiviral treatment will decrease the risk for infection transmission within dialysis units and reduce the occurrence of complications occurring after kidney transplantation.
References
- Yu TM, Lin CC, Shu KH, Chuang YW, Huang ST, et al. (2016) Increased risk of hepatic complications in kidney transplantation with chronic virus hepatitis infection: A nationwide population-based cohort study. Sci Rep 19(6): 21312.
- Trevizoli JE, Menezes R, Ribeiro VLF, Amorim R, Carvalho MB, et al. (2008) Hepatitis C is less aggressive in hemodialysis patients than in nonuremic patients. Clin J Am Soc Nephrol 3(5):1385-1390.
- Agarwal SK, Gupta SD (2015) Liver biopsy in patients on hemodialysis with hepatitis C virus infection: An important tool. Indian J Nephrol 25(3): 152-157.
- Boyd SD, Tracy L, Komatsu TE, Harrington PR, Viswanathan P, et al. (2017) US FDA perspective on elbasvir/grazoprevir treatment for patients with chronic hepatitis c virus genotype 1 or 4 infection. Clin Drug Investig 37(4): 317-326.
- Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, et al. (2016) Efficacy of direct-acting antiviral combination for patients with hepatitis c virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology 150(7):1590-1598.
- Sulejmani N, Jafri SM, Gordon SC (2016) Pharmacodynamics and pharmacokinetics of elbasvir and grazoprevir in the treatment of hepatitis C. Expert Opin Drug Metab Toxicol 12(3): 353-361.
- Deltenre P, Moreno C, Tran A, Ollivier I, Provôt F, et al. (2011) Antiviral therapy in haemodialysed HCV patients: efficacy, tolerance and treatment strategy. Aliment Pharmacol Ther 34(4): 454-461.
- Muñoz GR, Rincón D, Ahumada A, Hernández E, Devesa MJ, et al. (2017) Therapy with ombitasvir/paritaprevir/ritonavir plus dasabuvir is effective and safe for the treatment of genotypes 1 and 4 hepatitis C virus (HCV) infection in patients with severe renal impairment: A multicentre experience. J Viral Hepat 24(6): 464-471.
- Flisiak R, Janczewska E, Wawrzynowicz SM, Jaroszewicz J, Zarębska MD, et al. (2016) Real-world effectiveness and safety of Ombitasvir/ Paritaprevir/Ritonavir±dasabuvir±ribavirin in hepatitis C: AMBER study. Aliment Pharmacol Ther 44(9): 946-956.
© 2019 Moutaz D. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






