- Submissions

Full Text
Global Journal of Endocrinological Metabolism
U-500 Insulin: An Effective Treatment for Patients with Type 2 Diabetes and Severe Insulin Resistance
Nasser Mikhail*
Department of Medicine, Endocrine Division, USA
*Corresponding author: Nasser Mikhail, Department of Medicine, USA
Submission: February 20, 2019; Published: March 14, 2019

ISSN 2637-8019Volume3 Issue1
Abstract
Human regular insulin 500 (U-500) is a form of concentrated insulin that allows injection of high insulin doses with 5 times less volume compared with regular insulin. This concentrated insulin is indicated in patients with type 2 diabetes who require more than 200 units of insulin per day. Insulin U-500 displays both prandial and basal actions, and therefore can be injected as monotherapy in a convenient twicedaily regimen. Available data, mainly retrospective, suggest that insulin U-500 is effective, associated with better adherence, and decreased injection pain compared with non-concentrated insulins. Its main limitations are hypoglycemia and weight gain, and possibility of dosing errors. Randomized trials are needed to compare long-term efficacy and safety of insulin U-500 with other forms of insulin regimens.
Introduction
It is not uncommon that some patients with insulin-resistant type 2 diabetes, particularly morbidly obese, require large doses of insulin for glycemic control. Meanwhile, most insulin preparations are present in concentrations of 100 units/ml. Therefore, a patient who needs one insulin dose of more than 100 units has to use more than one injection. Insulin U-500 is a human regular insulin formulation that is 5 times as concentrated as the commonly used human regular insulin (U-100), i.e. U-500 contains 500 units of insulin per ml as opposed to 100 units/ml in non-concentrated insulins. This concentrated formulation allows the injection of large insulin doses of greater than 100units to be delivered in the fifth amount of volume of non-concentrated insulin.
Insulin U-500 was approved by the Federal Drug Administration (FDA) in the USA in 1994 for insulin-resistant patients with type 2 diabetes who require more than 200 units of insulin per day [1]. Because of rise in incidence of obesity in the USA, the use of U-500 has been increased approximately 10-fold from 2005 to 2013 [2]. The purpose of this article is to provide a mini-review on the pharmacokinetics, efficacy, safety, advantages and limitations of U-500 based on the available data and author’s experience.
Pharmacokinetics
The time action profile of regular U-500 insulin exhibits both prandial and basal effects [1]. Thus, mean onset and duration of action are 15min and 21 hours, respectively [2]. The peak plasma insulin levels occurred between 4 hours (50-unit dose) and 8hours (100-unit dose).
Concentrated insulins are absorbed slower than non-concentrated insulin [3]. Thus, when compared with traditional human regular insulin (U-100) at doses of 100 units in healthy obese subjects, time to peak concentration and time to maximum effect were significantly longer with U-500 than U100. In addition, maximum plasma concentrations (Cmax) of U-500 were significantly lower than U-100, and its duration of action was longer (21.5 hours versus 18.3 hours for U-100 leading to a flat time-action profile. Mean half-life after a single 100-unit dose is approximately 4.4h for U-500 versus 3.3 h for U-100 [4].
Available preparations of insulin U-500
U-500 is dispensed in a 3ml pen (1,500 units) or in a 20ml-vial (10,000 units), with maximum insulin doses delivered in a single injection of 300 and 250 units, respectively [1].
Efficacy of insulin U-500
Available data suggest that U-500 is effective, but the degree of efficacy is variable depending on several factors: doses, patients’ characteristics, baseline hemoglobin A1c (HbA1c). Unfortunately, there are no available randomized trials comparing U-500 with other types of insulin regimen. One randomized trial compared U-500 given bid with U500 given tid. After 24 weeks, there was no significant difference in HbA1c reduction, 1.12% and 1.22% in the TID and BID regimen, respectively [5].
Safety of insulin U-500
Similar to other insulins, the 2 most common adverse effects of U-500 are hypoglycemia and weight gain. Yet, because of lack of head to head comparison between U-500 and other insulins, the specific impact of U-500 on these 2 parameters is not clear. Hood et al. [5] found higher frequency of nocturnal hypoglycemia (blood glucose < 50mg/dl) in patients randomized to U-500 given BID compared with TID, 36% and 49%, respectively. Likewise, symptomatic hypoglycemia (blood glucose < 70mg/dl) occurred slightly more frequently among the BID group (92%) compared with TID group (90%), P=0.003. In the previous study, weight gain was 4.9kg and 5.4kg in the 2 groups of patients randomized to U-500 TID and BID, respectively.
Patient satisfaction
Patients who switched from multiple insulin injections (median 5 injections/day) to U-500 reported improvement in treatment burden, daily life and compliance, particularly with the BID regimen compared with the TID regimen. Moreover, there was significant improvement in injection site pain from baseline to end of trial at week 24 whether U-500 was given BID or TID [6].
Indications and initiation of insulin U-500
Candidates for U-500 are insulin-resistant patients with type 2 diabetes requiring more than 200 units of insulin/day. These patients are commonly morbidly obese and already receiving high number (5-7) of insulin injections/day. Before the conversion of prior insulin regimen to U-500, it is crucial to confirm the actual insulin doses because incomplete adherence to insulin therapy is frequent as result of increase injection burden. It is a common practice to reduce insulin daily doses by~20% or more when converting to U-500 to avoid hypoglycemia and to account for possible prior non-adherence [7]. Then, the U-500 total daily dose may be divided into 2 doses: 60% of the dose 30 min before breakfast and 40% 30min before dinner to decrease incidence of nocturnal hypoglycemia.
Use of U-500 in hospitals
The use of U-500 is not indicated in hospitalized patients to avoid dosing errors. When a patient taking U-500 is admitted to the hospital, it is safer to switch to a non-concentrated insulin regimen. Because food intake in hospitals is much less compared with home, it is recommended to decrease the initial total daily dose of insulin by 50% or more after hospital admission [8,9].
Cost of insulin U-500
The whole price sale of 20ml vial (10,000 units) of U-500 and the 3ml Kwik-pen (1,500 units) is approximately $1,200 and $303, respectively [10]. Although these prices are higher than regular insulin U-100, using a large healthcare claims database in the USA, Eby et al. [11] showed that treatment with insulin U-500 was associated with significant decreases in pharmacy and overall costs, and better adherence compared with treatment with high-dose (>200 units/day) of insulin U-100. Moreover, there was no significance difference between the 2 insulin regimens in hypoglycemia-specific costs and in resource utilization.
Limitations of U-500
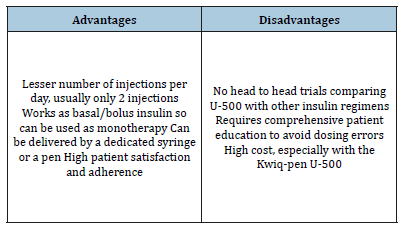
Dosing errors may occur if patient is not properly informed about the concept of U-500, and if U-500 is injected by syringes of non-concentrated insulin. Propensity of dosing errors has greatly diminished after the introduction of a dedicated syringe, specifically graduated for U-500 injection. This U-500 specific syringe was approved by the FDA and is commercially available since 2016. It is a 0.5ml green-capped syringe with bold markings in 5-unit increments allowing a maximum dose of 250 units at one injection [12]. In addition, in 2016, the FDA approved the U-500 R Kwik-pen by Eli Lilly [1]. This pen can provide up to 300 units per dose but is more expensive than the dedicated syringe. Advantages and disadvantages of U-500 are summarized in Table 1.
Table 1:Advantages and disadvantages of insulin U-500.
Conclusion
Insulin U-500 is the most concentrated available form of insulin allowing the reduction of injected volume of insulin by one-fifth. This insulin is a convenient treatment of patients with type 2 diabetes who are insulin-resistant and require high number of daily insulin injections. Compliance with this form of insulin is higher than non-concentrated insulin because of reduction in injection frequency to only 2 injections per day, its use a monotherapy, and decreased injection pain. Randomized trials should better define the efficacy, safety, and overall cost of U-500 compared with other insulin modalities.
References
- (2015) Humilin R U-500 (Insulin Human Injection). Lilly USA, LLC, Indianapolis, IN, 46285, USA.
- Wong M, Jiang D, Perez NM (2015) Trends in incident and prevalence use of human regular U-500 insulin over a 9-year period in the United States. Presented at: American Association of Clinical Endocrinologists (AACE) 24th Annual Scientific and Clinical Congress, May 13-17, Nashville, USA.
- Schloot NC, Hood RC, Corrigan SM, Panek RL, Heise T (2019) Concentrated insulins in current clinical practice. Diab Res Clin Pract 148: 93-101.
- De La Pena A, Riddle M, Morrow LA, Jiang HH, Linnebjerg H, et al. (2011) Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human U-100 insulin in healthy obese subjects. Diab Care 34(12): 2496-2501.
- Hood RC, Arakaki RF, Wysham C, Li YG, Settles JA, et al. (2015) Two treatment approaches for human regular U-500 insulin in patients with type 2 diabetes not achieving adequate glycemic control on highdose U-100 insulin therapy with or without oral agents: a randomized, titration-to- clinical trial. Endo Practice 21(7): 782-793.
- Kabul S, Hood RC, Duan R, Delozier AM, Settles J (2016) Patient-reported outcomes I transition from high-dose U-100 insulin to human regular U-500 insulin in severely insulin-resistant patients with type 2 diabetes: analysis of a randomized clinical trial. Health Quality Life Outcomes 14(1): 139.
- Bergen P, Kruger DF, Taylor AD, Eid WE, Bhan A, et al. (2017) Translating U-500 randomized trial evidence to the practice setting. A diabetes Educator/Expert prescriber team approach. Diab Educator 43(3): 311- 323.
- Paulus AO, Colburn JA, True MW, Beckman DJ, Davis RP, et al. (2016) Evaluation of total daily dose and glycemic control for patients taking U-500 regular insulin admitted to the hospital 22(10): 1187-1191.
- Kedia R, Desouza C, Smith LM, Shivaswamy V (2017) A retrospective review of insulin requirements in patients using U-500 insulin hospitalized to a Veterans Affairs Hospital 31(5): 874-879.
- Medpage Today. Price tag on old insulin skyrockets (2014). Internet accessed February 16 (2019).
- Eby EL, Wang P, Curtis BH, Xie J, Haldane DC, et al. (2013) Cost, healthcare resource utilization, and adherence of individuals with diabetes using U-500 or U-100 insulin: a retrospective database analysis. J Med Economics 16(4): 529-538.
- Shaw KF, Valdez CA (2017) Development and implementation of U-500 regular insulin program in a federally qualified health center. Clin Diab J 35(3): 162-167.
© 2019 Nasser Mikhail. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






