- Submissions

Full Text
Significances of Bioengineering & Biosciences
Polydopamine And Protein Based Nanoparticles: A Skin Inspired Biological Tool
Vincent Ball*
Université de Strasbourg, France
*Corresponding author: Vincent Ball, Institut National de la Santé et de la Recherche Médicale, Unité Mixte de Recherche 1121, 11 rue Humann, 67085 Strasbourg Cedex, France
Submission: December 03, 2018; Published: December 14, 2018

ISSN 2637-8078 Volume2 Issue5
Abstract
Inspiration from nature constitutes a new creative branch of materials science in general and of nanotechnology in particular. Inspired by the relatively well-defined size distribution of the eumelanin grains in the skin and by the presence of proteins on their shell, a strategy has been elaborated to produce a stable suspension of polydopamine nanoparticles capped with proteins to allow for many applications in nanomedicine and in biotechnology. Only proteins containing specific sequences in amino acid allow to control the size of the obtained nanoparticles in a protein/ dopamine ratio dependent manner. This mini review summarizes the recent progress in this field.
Keywords: Bioinspired materials; Eumelanin; Polydopamine@protein nanoparticles
Introduction
Eumelanin is the black pigment produced in the skin by specialized organelles localized in melanocytes, the melanosomes, and affords protoprotection [1]. A similar material, called neuromelanin allows protection against oxidative processes in the central nervous system [2,3]. These materials appear in the form of (nano) particles with a well-defined and hierarchical size distribution [4] and are issued from the oxidation of L-DOPA the hydroxylated form of L-tyrosine and from dopamine in the case skin eumelanin and neuromelanin respectively. However, when L-DOPA or dopamine are oxidized in solution in the presence of various oxidants, among which the oxygen dissolved in water (in basic solutions) or sodium periodate, an insoluble precipitate is obtained in addition to a useful coating on the reaction vessel. This coating is adherent to almost all known materials and constitutes hence a universal surface functionalization method [5] but at the prize of huge lost in active molecules.
Some trials have hence been attempted to oxidize dopamine (and other catecholamines) in controlled conditions by adding diverse molecules to the reaction medium in order to limit the aggregation of the formed oligomers with the final issue of precipitation. The synthesis using ammonia as a catalyst allows to produce stable nanoparticles [6,7], the addition of surfactants like sodium dodecyl sulfate or hexadecyltrimethyl ammonium bromide seems to restrict the formation of polydopamine in the surfactant vesicles [8]. Polymers like poly(vinyl alcohol) [9] and proteins from the egg white [10] and human serum albumin [11] were also found to allow to control the size of the particles issued from the oxidation of dihydroxyindole and dopamine respectively. These two last approaches seem very promising because the obtained eumelanin like particles incorporate some of the proteins used to control their size as the eumelanin grains from the skin do. These synthetic approaches are hence biomimetic in essence. But what makes proteins efficient in the control of the oxidation of catecholamines? Here again inspiration from nature appeared an efficient method. It is the aim of this mini review to describe how proteins can allow to control the oxidation and subsequent self-assembly of catecholamines, taking dopamine as a prototypal example.
Production of Stable and Size Controlled Eumelanin Like Nanoparticles in the Presence of Proteins
A novel source of biological inspiration helped to find some issues in the problem of understanding what kind of amino acids or what kind of conformational structures could interact specifically with dopamine to control its oxidation and self-assembly. Chemical and biochemical analysis revealed that the chromaffin vesicles excreted by the adrenal gland are simultaneously rich in catecholamines and in proteins like chromogranin A (CgA) and B (CgB) [12] which mature upon controlled proteolysis to produce a repertoire of peptides having a broad range of biological activities like antimicrobial properties [13]. Most often, catecholamines are protected from oxidation in such vesicles. But occasionally, in specific metabolic conditions, an oxidative stress for instance, the chromaffin vesicles turn brownish implying catecholamine oxidation [14]. The possibility to control the oxidation of catecholamines leads to the assumption that among the pool of peptide sequences issued from CgA some of them should play a role in the control of the oxidation process and self-assembly of catecholamines into eumelanin like nanoparticles.
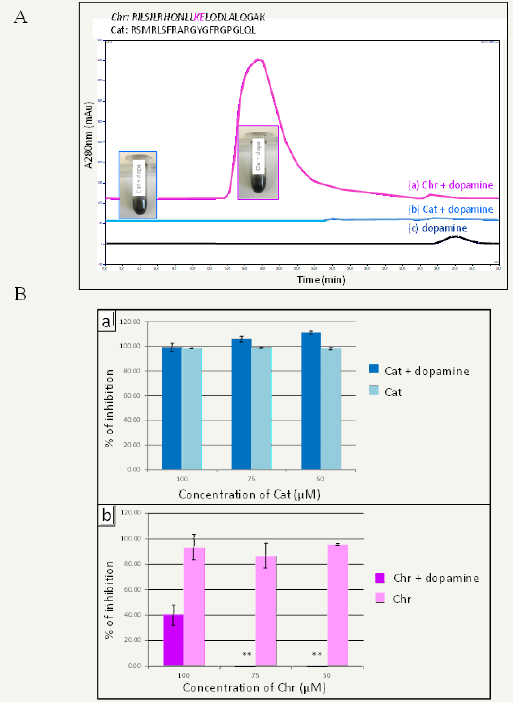
The identification of such short oligo-peptides (10-20 amino acids) should greatly help in understanding the role played by proteins in the controlled self-assembly of eumelanin like materials in nanoparticles. Using size exclusion chromatography on dopamine-peptide mixtures after 16h of oxidation, we found that certain mixtures lead to peaks corresponding to molecular masses of a few hundred thousands of g.mol-1 whereas other mixtures lead to elution in the solvent front corresponding to large polydopaminepeptide particles. Knowing the amino acid sequence of the peptides allowing to control the size of polydopamine aggregates, we came to the assumption that peptides containing the KE diad of amino acids allow to control the self-assembly of polydopamine in solution under oxidative conditions [15]. Figure 1 shows the example of Chromofungin (KE containing) and Cateslytin (no KE in the amino acid sequence) interacting with dopamine in oxidizing conditions. Both of these peptides have an antimicrobial activity but the antimicrobial effect of chromofungin, the KE containing peptide, is lost in contact with dopamine and after its oxidation (Figure 1B). This strongly suggests that Chromofiungin binds to the obtained polydopamine based particles.
Figure 1:Size exclusion chromatography profiles of oxidized dopamine in the presence of Tris buffer (pH = 8.5) alone and in the presence of Chromofungin (Chr) and Cateslytin (Cat). The pictures displayed in the inset show that polydopamine formation occurs in all cases after 16h in the presence of O2 from the air. The detection wavelength was set at 280nm. The sequence of both peptides is displayed on the top.
Figure 1B: Evolution of bacterial growth inhibition as a function of the peptide concentration (Cat in row a, Chr in row b) in the absence and in the presence of dopamine (the peptide / dopamine ratio was constant and equal to 1/10 in all experiments). The ** symbol means that the inhibition is statistically not different from zero. Modified from ref.[15] with authorization.
To confirm the hypothesis of the control exerted by KE diads, the investigation was then continued with short peptides containing the KE diad or peptides containing both amino acids spaced by one or two glycine (G) spacers. It was found that only one G between K and E suppressed all control over the size of polydopamine aggregates. In this case precipitates are formed during dopamine oxidation. To understand the influence of the KE diad on dopamine oxidation, molecular dynamics simulations were performed and it appeared that L-glutamic acid forms hydrogen bonds with the two hydroxyl groups of dopamine whereas L-lysine establishes cation-π interactions with the aromatic ring of dopamine (Figure 2).
Figure 2:Top part: Population analysis of the (N-GGKEGG-C (left) and N-GGKGEGG-C (right) peptides, as a function of the distance between the center of mass (CoM) of the benzene ring and the N atom of the NH3+ group of the lysine residue and between the C atom of the COO- of the glutamic acid ( E) residue and the center of mass of both –OH groups of dopamine. Green colored regions correspond to populations that are often observed whereas blue colored regions corresponds to populations that are seldomly observed. White colored regions population that are never observed.
Bottom part: Typical snapshot of the dopamine – N-GGKEGG-C pair corresponding to the 2 most observed populations. Reproduced from ref. [15].
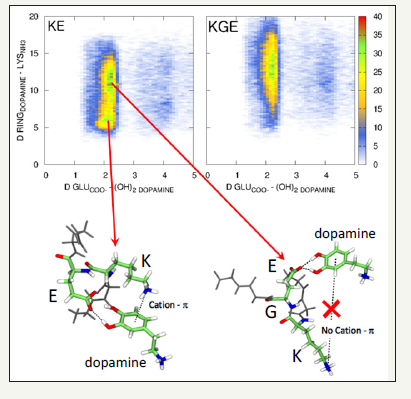
Figure 3:Evolution of the hydrodynamic radius of polydopamine particles after 24h of oxidation at pH = 8.5 in the presence of alkaline phosphatase (ALP) at different concentrations. The initial dopamine concentration was of 2mg.mL-1 in all experiments.
Figure 3B: Deposition of polydopamine on quartz plates monitored at λ=500 nm as a function of the oxidation time at pH = 8.5: in the absence of Alp ( ) and in the presence of Alp at 0.1 ( ) and 0.5 ( ) mg.mL-1. As a control experiment Alp was also preadsorbed during 1h from a solution at 1mg.mL-1 before the beginning of dopamine oxidation but in the absence of ALP ( ). Modified from ref. [15] with authorization.
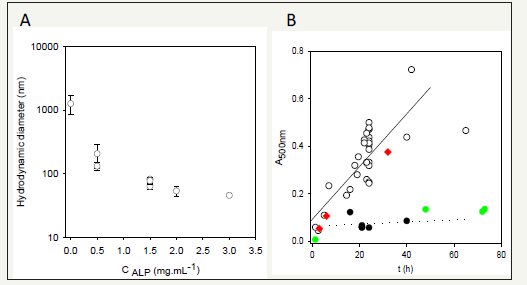
This knowledge allowed us to test the validity of the assumption that the KE diad present in proteins exerts an influence on the oxidation and self-assembly of dopamine. Indeed we found that KE containing proteins like alkaline phosphatase (Figure 3A) and human fibrinogen allow to produce polydopamine particles of controlled size with an hydrodynamic diameter decreasing with an increase in the initial protein/dopamine ratio [15]. Looking back to human serum albumin, which is able to control the size of polydopamine particles, it contains indeed several KE diads. In addition to the size control of polydopamine nanoparticles, those KE containing proteins, inhibit film deposition on the surface of the reaction beaker (Figure 3B). On the other hand, other proteins like hen egg white lysozyme and bovine α-lactalbumin do not allow to control the size of polydopamine in the suspension and do not hinder its deposition on the beaker walls: they act as spectators in the oxidation-self-assembly of dopamine and do not contain KE, as expected from the previous results.
Those polydopamine@protein nanoparticles keep, at least partly, the biological activity of the used protein as exemplified with alkaline phosphatase and a glucose oxidase+peroxidase mixture. The obtained nanoparticles could also be deposited on surfaces in a layer-by-layer manner in alternance with the polycationic poly (ally amine hydrocholoride) [15]. The obtained films displayed a stable enzymatic activity for prolonged duration.
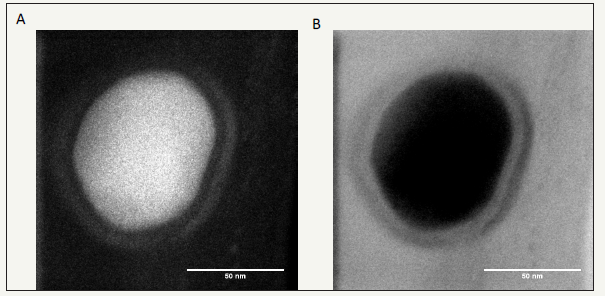
Finally, the polydopamine synthesis time could be reduced from 24 to 6h by replacing the dissolved oxygen as the oxidant by sodium periodate without affecting nor the particles size neither their biological activity [16]. The particles’ size can be controlled down to 40-50nm (Figure 4) and reproduced in a twostep synthetic pathway: oxidation of the dopamine-protein mixture followed by dialysis against the desired buffer to remove non-incorporated proteins and small oligomers of oxidized dopamine.
Figure 4:High resolution TEM images acquired simultaneously in high angle annular dark field (HAADF, left) and bright field (BF, right) modes of polydopamine@ALP nanoparticles obtained after 6h of oxidation of a 10mM dopamine solution + 1 mg.mL-1 ALP in the presence of 20mM NaIO4. The particles synthesis was followed by extensive dialysis against 50mM Tris buffer at pH = 8.5. Reproduced from ref. [16].
All the obtained PDA@protein nanoparticles displayed extremely low cytotoxicity up to high concentration levels [15]. In a totally different approach the 6 tripeptides containing L-tyrosine, L-phenylalanine and L-aspartic acid where self-assembled in oxidizing conditions to yield nanostructures with sequence encoded size and structures [17].
All these investigations highlight that catechols and catecholamines in interaction with proteins may constitute a fantastic tool in the design of nanomaterials with full biological functionality and extremely low cytotoxicity. All these materials are reminiscent of eumelanin grains found in the skin or in chromaffin vesicles. As such they already find interesting applications as described in the next section.
Potential Applications of Eumelanin like Nanoparticles
As eumelanin in the skin, the polydopamine@protein nanoparticles absorb light over the whole UV-vis spectrum and up to the infrared part of the spectrum with a very low fluorescence quantum yield [18]. Indeed the absorbed photons are mainly converted in heat a property which can be used for applications in photothermal therapy [6,19] even for real life biological applications because eumelanin and PDA are still able to absorb light in the near infrared window (however with much less efficiency than UV-light) for which biological tissues are partly transparent.
Owing to their antioxidant properties those catecholaminebased nanoparticles are also efficient free radical scavengers [20]. In the spirit of bio-inspired materials, the PDA nanoparticles can be used to build-up films displaying structural colours as in the feathers of many birds [21]. The suspensions of PDA@protein nanoparticles are very stable during their storage and constitute hence an ink which may easily printed using 3D printers.
Conclusion
Nanoparticles made from the oxidation of catecholamine – protein mixtures or from polypeptides containing oxidizable amino acids are synthetic analogues of eumelanin grains in the skin and constitute new tools in nanomedicine. Further research is however required to better understand the influence exerted by amino acids sequences in the control of the oxidation-self assembly processes allowing to obtain such nanoparticles. It is likely that other amino acid sequences, in a similar case as the several amino acid sequences implied in cell adhesion, can exert specific interactions with catecholamines during their oxidation. The discovery of those sequences may certainly be facilitated using a biomimetic approach as the one presented in this article.
References
- Brenner M, Hearing VJ (2008) The Protective Role of Melanin Against UV Damage in Human Skin. Photochem & Photobiol 84(3): 539-549.
- Zecca L, Gallorini M, Schünemann V, Trautwein AX, Gerlach M, et al. (2001) Iron, Neuromelanin and Ferritin Content in the Substantia Nigra of Normal Subjects at Different Ages: Consequences for Iron Storage and Neurodegenerative Processes. J Neurochem 76(6): 1766-1773.
- Double KL (2000) Structural Characteristics of Human Substantia Nigra Neuromelanin and Synthetic Dopamine Melanins. J Neurochem 75: 2583-2589.
- Clancy CMR, Nofsinger JB, Hanks RK, Simon JD (2000) A Hierarchical Self-Assembly of Eumelanin. J Phys Chem B 104(33): 7871-7873.
- Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 318(5849): 426-430.
- Liu H, Ai K, Liu J, Deng M, He Y, et al. (2013) Dopamin-melanin colloidal nanospheres; an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv Mater 25(9): 1353-1359.
- Wang Z, Wang K, Zhang Y, Jiang Y, Lu X, et al. (2016) Protein affinitive polydopamine nanoparticles as an efficient surface modification strategy for versatile porous scaffolds enhancing tissue regeneration. Part Part Syst Charac 33(2): 89-100.
- Ponzio F, Bertani Ph, Ball V (2014) Role of Surfactants in the Control of Dopamine-Eumelanin Particle Size and in the Inhibition of Film Deposition at Solid-Liquid Interfaces. J Colloid & Interf Sci 431: 176-179.
- Arzillo M, Mangiapia G, Pezzella A, Heenan RK, Radulescu A, et al. (2012) Eumelanin Buildup on the Nanoscale: Aggregate Growth/Assembly and Visible Absorption Development in Biomimetic 5,6-dihydroxyindole Polymerization Biomacromolecules 13(8): 2379-2390.
- Della Vecchia F, Cerruti P, Gentile G, Errico ME, Ambrogi V, et al. (2015) Artificial Biomelanin: Highly Light Absorbing Nano-Sized Eumelanin by Biomimetic Synthesis in Chicken Egg White. Biomacromolecules 15(10): 3811-3816.
- Chassepot A, Ball V (2014) Human Serum Albumin and Other Proteins as Templating Agents for the Synthesis of Nanosized Dopamine-Eumelanin. J Colloid & Interf Sci 414: 97-102.
- Crivellato E, Nico B, Ribatti D (2008) The Chromaffin Vesicle: Advances in Understanding the Composition of a Versatile, Multifunctional Secretary Organelle. The Anat Rec 291(12): 1587-1602.
- Aslam R, Atindehou M, Lavaux T, Haïkel Y, Schneider F, et al. (2012) Chromogranin A-Derived Peptides are Involved in Innate Immunity. Curr Med Chem 19(24): 4115-4123.
- Ogata T, Ogata A (1923) Über die Henlesche Chromreaktion der Sogennaten Chromaffinen Zellen und den Mikroskopischen Nachweis des Adrenalins. Ziegler Beitr 71: 376-387.
- Bergtold C, Hauser D, Chaumont A, El Yakhlifi S, Mateescu M, et al. (2018) The KE sequence in polypeptides and in proteins allows for a specific control of nanosized polydopamine formation. Biomacromolecules 19: 3693-3704.
- El Yakhlifi S, Ihiawakrim D, Ersen O, Ball V (2018) Enzymatically active polydopamine@alkaline phosphatase nanoparticles produced by NaIO4 oxidation of dopamine. Biomimetics 3(4): 36.
- Lampel A, McPhee SA, Park KA, Scott GG, Humagain S, et al. (2017) Polymeric Peptide Pigments with sequence-encoded properties. Science 356(6342): 1064-1068.
- Meredith P, Sarna T (2006) The Physical and Chemical Properties of Eumelanin. Pig Cell Res 19(6): 572-594.
- Han L, Zhang Y, Lu X, Wang K, Wang Z, et al. (2016) Polydopamine Nanoparticles Modulating Stimuli Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Applied Mater & Interf 8(42): 29088- 29100.
- Ju KY, Lee Y, Lee S, Park SB, Lee JK (2011) Bioinspired Polymerization of Dopamine to Generate Melanin-like Nanoparticles Having an excellent free-radical Scavenging Property. Biomacromolecules 12(3): 625-632.
- Xiao M, Li Y, Allen MC, Deheyn DD, Yue X, et al. (2015) Bio-Inspired Structural Colours Produced via Self-Assembly of Synthetic Melanin Nanoparticles. ACS Nano 9(5): 5454-5460.
© 2018 Vincent Ball. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






